Key Points
Complement activation is not required for development of thrombotic microangiopathy and HUS induced by EHEC Shiga toxins in nonhuman primates.
Complement is an important defense mechanism, and benefits or risks of therapeutic inhibition should be studied further for this infection.
Abstract
Enterohemorrhagic Escherichia coli (EHEC) produce ribosome-inactivating Shiga toxins (Stx1, Stx2) responsible for development of hemolytic uremic syndrome (HUS) and acute kidney injury (AKI). Some patients show complement activation during EHEC infection, raising the possibility of therapeutic targeting of complement for relief. Our juvenile nonhuman primate (Papio baboons) models of endotoxin-free Stx challenge exhibit full spectrum HUS, including thrombocytopenia, hemolytic anemia, and AKI with glomerular thrombotic microangiopathy. There were no significant increases in soluble terminal complement complex (C5b-9) levels after challenge with lethal Stx1 (n = 6) or Stx2 (n = 5) in plasma samples from T0 to euthanasia at 49.5 to 128 hours post-challenge. d-dimer and cell injury markers (HMGB1, histones) confirmed coagulopathy and cell injury. Thus, complement activation is not required for the development of thrombotic microangiopathy and HUS induced by EHEC Shiga toxins in these preclinical models, and benefits or risks of complement inhibition should be studied further for this infection.
Introduction
Shiga toxin-producing enterohemorrhagic Escherichia coli (EHEC) is an emerging food- and water-borne pathogen.1 The E coli O157:H7 is the most common strain, and its ribosome inactivating Shiga toxins (Stx1 and Stx2) injure receptor-bearing endothelial cells, particularly in renal glomeruli. Hemolytic uremic syndrome (HUS) is a clinically important complication in 5% to 15% of these patients, characterized by hemolytic anemia, thrombocytopenia, and thrombotic microangiopathy, often resulting in severe acute kidney injury (AKI) necessitating dialysis. Antibiotics increase HUS risk,2 and this pathogen is the leading cause of acute renal failure in otherwise healthy US children.
The clinical presentation of EHEC-HUS overlaps that of atypical HUS (aHUS), a rare disease induced by genetic abnormalities resulting in unchecked alternative complement pathway activation.3 Immuno-inhibition of complement C5 activation in aHUS patients with Eculizumab (Soliris) reduces levels of inflammatory complement mediators and the terminal complement complex (TCC, soluble C5b-9) and normalizes clinical indicators.4 The clinical similarity of these syndromes has led to considerable discussion in the EHEC field about whether Stx activities activate complement, which then becomes a major driving force for HUS development. In vitro and murine data support complement activation,5-7 but in vitro data arise from toxin challenges ∼500 000 times higher than the 5 to 20 pg/mL Stx levels observed in infected children.8 Eculizumab was seemingly beneficial in 3 children with severe EHEC-HUS,9 but apparent efficacy may have been coincident with natural recovery as suggested by already rising platelets and falling lactate dehydrogenase levels. Despite these indicators, a direct exploration of whether Stx-induced complement activation is responsible for HUS has not been done. Our nonhuman primate models of endotoxin-free Stx1 and Stx2 challenge present with the full spectrum of human EHEC-HUS including hematology, physiology, and inflammation responses,10,11 with glomerular thrombotic microangiopathy.12 Here we examined whether complement was activated in the Stx-challenged baboons during the development of HUS and acute renal failure. We quantified d-dimer as a marker of fibrinolysis, as well as cell injury markers HMGB1 and histones.
Study design
Baboon samples
Methods and characterization of the nonhuman primate (juvenile Papio baboons, 4-6 kg) challenges with lethal Stx1 (100 ng/kg) or Stx2 (50 ng/kg) have been described.10,11 All animals developed HUS with thrombotic microangiopathy and progressive loss of renal function. Animal studies were performed under the oversight of the Institutional Animal Care and Use Committee of the Boston University School of Medicine.
Enzyme-linked immunosorbent assays
Baboon EDTA-plasma or urine (Foley catheter; 20-minute timed samples after bladder purge) stored at −80°C were used. TCC was quantified using the Human Terminal Complement Complex enzyme-linked immunosorbent assay (ELISA) kit (Hycult Biotech, Plymouth Meeting, PA). d-dimer was quantified using the Asserachrom D-DI kit (Diagnostica Stago, Parsippany, NJ). HMGB1was quantified using HMGB1 ELISA (IBL International, Hamburg, Germany) and histones by using Cell Death Detection ELISA Plus (Roche, Indianapolis, IN). Stored EDTA-plasma from bacteremic baboons challenged intravenously with sublethal 5 × 109 CFU/kg E coli B7 O86a:K61 (SLEC; not toxigenic)13 or lethal 3 × 109 CFU/kg Bacillus anthracis Sterne strain 34F2 (vaccine strain)14 were positive controls. Data were analyzed for differences between groups using the Student t test, assuming equal variance.
Results and discussion
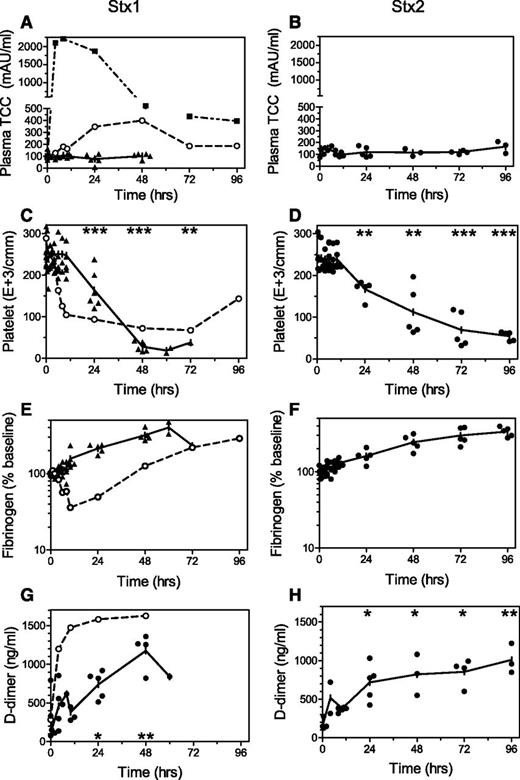
Bacteremia is rare in patients with EHEC infection, and the Shiga toxins are widely acknowledged as the primary mediators of organ injury.15,16 Our nonhuman primate models are the only animal models to date that present with full spectrum HUS induced by only Stx challenge. Some differences are observed between the toxins with respect to timing and inflammation10 or renal pathology,12 but the classic triad of hemolytic anemia, thrombocytopenia, and thrombotic microangiopathy with AKI is a shared response after Stx1 or Stx2. Given the success of complement inhibition in aHUS patients, we measured soluble TCC in our Stx-HUS models to determine whether complement is activated and, if so, when. There were no significant increases in soluble TCC levels in animals after lethal challenge with Stx1 (Figure 1A; n = 6) or Stx2 (Figure 1B; n = 5) until the time of euthanasia at 49.5 to 128 hours after challenge. Platelet levels decreased (Figure 1C-D), and fibrinogen levels were steady or increased (Figure 1E-F) as expected during development of HUS. Renal glomerular thrombotic microangiopathy was observed by pathology evaluation after necropsy,12 and progressively increasing d-dimer levels (Figure 1G-H) confirm coagulation activation and subsequent fibrinolysis. The lack of complement activation was surprising, given the known crosstalk between coagulation and complement pathways17 and markers of complement activation in some EHEC-HUS patients.6,7 To confirm integrity of the assays with baboons, the well-characterized baboon model of E coli bacteremia sepsis with disseminated intravascular coagulation13,18,19 was evaluated similarly. Sublethal challenge with this E coli strain induced complement activation and disseminated intravascular coagulation as judged by thrombocytopenia, fibrinogen consumption, and rapid increases in d-dimer (Figure 1A,C,E,G). Complement inhibition after a high dose of this E coli strain in baboons significantly reduces the consumptive coagulopathy and inflammation.19 Similarly, soluble TCC in a baboon challenged with intravenous Gram-positive attenuated Bacillus anthracis14 peaked at 2,204.99 mAU/mL by 10 hours after challenge and slowly declined over the next 4 days (Figure 1A).
Changes in complement and coagulation activation markers. Stored timed EDTA-plasma samples from baboons challenged with intravenous 100 ng/kg Stx1 (▲, left; n = 6) or 50 ng/kg Stx2 (●, right; n = 5) were evaluated by ELISA for levels of (A-B) soluble TCC (C5b-9). After toxin, the (C-D) thrombocytopenia, (E-F) steady or increasing fibrinogen levels (% change from T0), and (G-H) increasing d-dimer levels are consistent with development of hemolytic uremic syndrome and AKI in these models. In contrast, bacteremia induced by intravenous challenge with pathogenic E coli (○, A,C,E,G) or attenuated B anthracis (dashed ▪, A), resulted in rapid and robust rises in complement activation accompanied by increased d-dimer and consumption of platelets and fibrinogen, consistent with disseminated intravascular coagulation. Means are plotted with individual animal values to show variability between animals. Significant differences from T0 (mean of each Stx group): *P < .05, **P < .01, ***P < .001.
Changes in complement and coagulation activation markers. Stored timed EDTA-plasma samples from baboons challenged with intravenous 100 ng/kg Stx1 (▲, left; n = 6) or 50 ng/kg Stx2 (●, right; n = 5) were evaluated by ELISA for levels of (A-B) soluble TCC (C5b-9). After toxin, the (C-D) thrombocytopenia, (E-F) steady or increasing fibrinogen levels (% change from T0), and (G-H) increasing d-dimer levels are consistent with development of hemolytic uremic syndrome and AKI in these models. In contrast, bacteremia induced by intravenous challenge with pathogenic E coli (○, A,C,E,G) or attenuated B anthracis (dashed ▪, A), resulted in rapid and robust rises in complement activation accompanied by increased d-dimer and consumption of platelets and fibrinogen, consistent with disseminated intravascular coagulation. Means are plotted with individual animal values to show variability between animals. Significant differences from T0 (mean of each Stx group): *P < .05, **P < .01, ***P < .001.
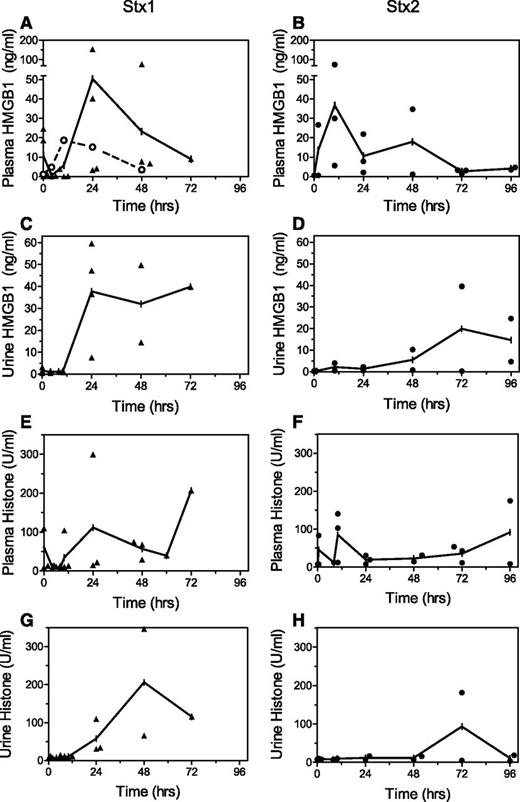
Markers of cell damage also were evaluated in the Stx-challenged baboons to confirm systemic cytotoxic activities. HMGB1 and histones are damage-associated molecular patterns, which are released into the circulation by dead or dying cells. They can engage receptors on distant cells and are proinflammatory in murine and baboon sepsis models.20 Both damage-associated molecular patterns were detected in baboon plasma and urine after Stx challenge (Figure 2), with generally earlier rises after Stx1, consistent with earlier increases in inflammation cytokines and chemokines.10
Cell injury markers. Challenge of baboons with lethal Stx1 (▲, left) or Stx2 (●, right) led to increases in plasma and urine levels of (A-D) HMGB1 and (E-H) histones. Stx1 led to earlier and higher levels, consistent with a more proinflammatory environment after this toxin.10 Plasma HMGB1 increased modestly after intravenous challenge with pathogenic E coli (○, A), returning to baseline values within 2 days after this sublethal challenge. Means are plotted with individual animal values to show variability.
Cell injury markers. Challenge of baboons with lethal Stx1 (▲, left) or Stx2 (●, right) led to increases in plasma and urine levels of (A-D) HMGB1 and (E-H) histones. Stx1 led to earlier and higher levels, consistent with a more proinflammatory environment after this toxin.10 Plasma HMGB1 increased modestly after intravenous challenge with pathogenic E coli (○, A), returning to baseline values within 2 days after this sublethal challenge. Means are plotted with individual animal values to show variability.
Collectively, the data show that the complement pathway is not activated to any great extent in this animal model of HUS despite challenge with sufficient Stx to induce coagulation, fibrinolysis, cell injury, and ultimately death. Mice infected with Citrobacter rodentium expressing Stx2 develop EHEC-like intestinal adhesion and inflammatory lesions with Stx-induced AKI, but also do not show evidence of complement activation.16 However, complement activation is reported in some EHEC-HUS patients. It is not clear whether Eculizumab was effective during the German 2011 outbreak,21,22 but this was an enteroaggregative E coli O104:H4 strain that acquired both Stx2 expression and unusual virulence in young adults with differing clinical presentation from more typical enterohemorrhagic strains.23 Our baboon HUS models are induced by Stx challenge and not an enteric bacterial infection, and bacterial translocation from the intestines is not observed in the baboons.24 However, there is considerable intestinal injury in EHEC patients that may contribute to complement activation. The colon can be affected with edema, hemorrhage, and leukocytosis, consistent with the hemorrhagic colitis that is often seen preceding HUS, but severity may vary widely between patients. Although our data do not support a major role for activation of complement during Stx-induced HUS pathogenesis, other host or bacterial virulence factors25 may be important, either alone or in combination with the bacterial toxins. Complement is a fundamental bacterial defense mechanism, and further research is warranted to judge therapeutic risks or benefits of modulating this arm of innate immunity in EHEC patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge Valta Freeman, Scott Freeman, and Diann Debord for technical assistance and Lyndianne Joseph for administrative assistance.
This work was supported by National Institutes of Health, National Institute of Allergy and Infectious Diseases grants RO1 AI058107, UO1 AI1075386, and U19 AI062629 (S.K.) and R42AI062095 (S. Opal, subcontract to S.K.). Animals were obtained from Baboon Research Resources, supported by National Institutes of Health, National Center for Advancing Translational Sciences grant P40OD010988 (G. White).
Authorship
Contribution: B.C.L. performed the immunoassays; S.K. and D.J.S.-K. conceived the hypothesis and designed the experiments; and B.C.L., C.L.M., C.S.L., D.J.S.-K., and S.K. contributed to data interpretation, wrote the manuscript, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shinichiro Kurosawa, Department of Pathology and Laboratory Medicine, Boston University School of Medicine, 670 Albany St, Boston, MA 02118; e-mail: kurosawa@bu.edu; and D. J. Stearns-Kurosawa, Department of Pathology and Laboratory Medicine, Boston University School of Medicine, 670 Albany St, Boston, MA 02118; e-mail: dstearns@bu.edu.