Key Points
Platelet PDI regulates αIIbβ3 integrin activation without affecting platelet activation and inside-out integrin signaling.
Platelet PDI is essential for platelet accumulation but not for fibrin generation and hemostasis in mice.
Abstract
Protein disulfide isomerase (PDI) derived from intravascular cells is required for thrombus formation. However, it remains unclear whether platelet PDI contributes to the process. Using platelet-specific PDI–deficient mice, we demonstrate that PDI-null platelets have defects in aggregation and adenosine triphosphate secretion induced by thrombin, collagen, and adenosine diphosphate. Such defects were rescued by wild-type but not mutant PDI, indicating that the isomerase activity of platelet surface PDI is critical for the regulatory effect. PDI-deficient platelets expressed increased levels of intracellular ER protein 57 (ERp57) and ERp72. Platelet PDI regulated αIIbβ3 integrin activation but not P-selectin exposure, Ca2+ mobilization, β3–talin1 interaction, or platelet spreading on immobilized fibrinogen. Inhibition of ERp57 further diminished αIIbβ3 integrin activation and aggregation of activated PDI-deficient platelets, suggesting distinct roles of PDI and ERp57 in platelet functions. We found that platelet PDI is important for thrombus formation on collagen-coated surfaces under shear. Intravital microscopy demonstrates that platelet PDI is important for platelet accumulation but not initial adhesion and fibrin generation following laser-induced arteriolar injury. Tail bleeding time in platelet-specific PDI–deficient mice were not significantly increased. Our results provide important evidence that platelet PDI is essential for thrombus formation but not for hemostasis in mice.
Introduction
Platelets play a central role in hemostasis and atherothrombosis. Following vascular injury, platelets rapidly adhere to activated endothelial cells and/or subendothelial matrix proteins such as collagen and von Willebrand factor through receptor–ligand interactions.1 Subsequently, activated platelets expose P-selectin from α-granules to the plasma membrane and release other granular molecules such as adenosine diphosphate (ADP), which activates other platelets and facilitates αIIbβ3 integrin–mediated platelet accumulation at the site of vascular injury. Although it is not fully understood how integrin function is regulated, it has been postulated that thiol rearrangement in integrins could be one of the regulatory mechanisms.2-4 Previous studies showed that αIIbβ3 integrin has an endogenous isomerase activity and exposes free sulfhydryl groups during platelet activation.4-6 Consistently, reducing agents such as reduced glutathione and cysteine affect platelet aggregation.2,7,8 Using αIIbβ3 integrin with mutations on Cys residues, Mor-Cohen et al9 reported that different disulfide bonds in the β3 subunit change the structure and function of αIIbβ3 integrin. Moreover, disruption of the disulfide bonds of Cys5-Cys435 or Cys663-Cys687 on the β3 subunit resulted in the conformational change of αIIbβ3 integrin into its active state.10,11 These results suggest that thiol modification could be important for the conformational change of αIIbβ3 integrin during the process of platelet activation and aggregation.
Protein disulfide isomerase (PDI) catalyzes disulfide bond exchange during protein synthesis in the endoplasmic reticulum (ER), where two active CGHC thioredoxin motifs in PDI are essential for oxidoreductase activity.12 In addition to its critical role in the ER, there is growing evidence that PDI is localized on the cell surface. Treatment of platelets with anti-PDI antibodies that block its enzymatic activity has been reported to significantly inhibit integrin αIIbβ3- and α2β1-mediated platelet function.13,14 Real-time intravital microscopic analysis and tail bleeding time assays in live mice demonstrated that PDI derived from intravascular cells is required for both thrombogenesis and hemostasis.15 Furthermore, recent studies have shown that purified PDI directly binds to αIIbβ3 integrin and that both platelet and endothelial cell β3 integrins are crucial for rapid accumulation of extracellular PDI at the site of laser-induced arteriolar injury, implying that PDI binds to β3 integrins and regulates their function in vivo.16 Nevertheless, it remains unknown how cell-specific PDI contributes to thrombus formation at the site of vascular injury.
Using megakaryocyte- and platelet-specific PDI–deficient mice, we demonstrate that the isomerase activity of platelet surface PDI is important for regulating platelet aggregation and adenosine triphosphate (ATP) secretion but not for inducing P-selectin exposure, Ca2+ mobilization, protein phosphorylation, β3–talin1 interaction, or platelet spreading. Studies with PDI-null platelets and blocking antibodies against ER protein 57 (ERp57) suggest that PDI and ERp57 could play a distinct role in regulating platelet functions. Intravital microscopic analysis shows that platelet PDI regulates thrombus growth but not initial platelet adhesion and fibrin generation at the site of arteriolar injury in live mice. Tail bleeding time and blood loss did not significantly increase in platelet-specific PDI–deficient mice, compared with control mice. These results indicate that the isomerase function of platelet PDI is restricted to thrombus formation and not essential for hemostasis in mice.
Materials and methods
Mice
Wild-type (WT), platelet factor 4-Cre (PF4-Cre),17 and β3 knockout (KO) mice18 were purchased from The Jackson Laboratory (Bar Harbor, ME). Platelet-specific PDI conditional KO (CKO) mice were generated by crossing PDI floxed mice19 with PF4-Cre mice (supplemental Methods, available on the Blood web site; see the supplemental Materials link at the top of the online article). The University of Illinois Institutional Animal Care and Use Committee approved all animal care and experimental procedures.
Approval to collect blood samples was obtained from the University of Illinois-Chicago review board. This study was conducted in accordance with the Declaration of Helsinki.
Reagents
Isolation of mouse platelets, neutrophils, and endothelial cells
The detailed methods to isolate mouse platelets, neutrophils, and endothelial cells are presented in the supplemental Methods.
Binding of wtPDI and dmPDI to mouse platelets
Recombinant wild-type PDI (wtPDI) and mutant PDI (dmPDI), in which the active site CGHC residues were mutated into SGHS residues, were generated as described previously.19 Platelets were preincubated with 50 μg/mL His-tagged wtPDI or dmPDI in HEPES-Tyrode buffer containing 1 mM EGTA for 5 minutes at room temperature. Platelets were then treated with or without 0.05 U/mL thrombin for 5 minutes at 37°C under constant stirring at 1000 rpm. The reactions were stopped with 50 μM PPACK (d-phenylalanyl-l-prolyl-l-arginine chloromethyl ketone). After washing twice with HEPES-Tyrode buffer or carbonate buffer (0.1 M Na2CO3, pH 9.0), platelets were incubated with Dylight 488–conjugated anti-polyHis antibodies and analyzed by flow cytometry.
Platelet spreading and flow chamber assay
Platelet aggregation assay, calcium mobilization, flow cytometric analysis, immunoblotting, immunoprecipitation, intravital microscopy, and tail bleeding time
Detailed methods are presented in the supplemental Methods.
Statistics
Data analysis was performed using the statistical software GraphPad Prism 5. Statistical significance was assessed by ANOVA (analysis of variance) or Kruskal-Wallis test for comparison of multiple groups or Student t-test for comparison of 2 groups. A P value < .05 was considered significant.
Results
Platelets of PDI CKO mice do not express PDI but have increased levels of intracellular ERp57 and ERp72
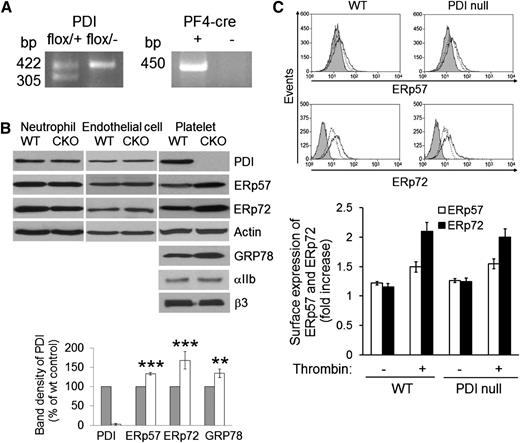
To study the role of platelet PDI in thrombosis and hemostasis, platelet-specific PDI–deficient mice were generated using the recombination strategy mediated by PF4-Cre transgenic mice as previously described.17 Littermate WT (PDIflox/+) control and PDI CKO (PDIflox/−; PF4-Cre) mice were confirmed by PCR analysis (Figure 1A). As confirmed by immunoblotting, PDI expression was ablated >97% in platelets of the CKO mice (Figure 1B). In contrast, the content of PDI in bone marrow neutrophils and heart endothelial cells of the CKO mice was normal. Flow cytometric analysis using anti-CD31 and anti–Flk-1 antibodies demonstrated that the purity of endothelial cells was 65% to 85% (supplemental Figure 1). Further, the protein amount of CD31 and Flk-1 in lysates of endothelial cells isolated from WT and CKO mice was similar. Disruption of the PDI gene did not alter the protein expression of actin, αIIb, and β3 in platelets (Figure 1B), and no difference was observed in the surface expression of αIIbβ3 integrin and glycoprotein Ibα between control and PDI-null platelets (supplemental Figure 2). The number of circulating blood cells was not different between WT and PDI CKO mice (data not shown). Because PDI has at least 21 family members in mammals,25 we examined whether PDI gene deletion affects the expression of other PDI family members. Indeed, the expression of PDI family members ERp57 and ERp72 and an ER stress marker, GRP78, was significantly upregulated in PDI-null platelets compared with WT platelets (Figure 1B). As analyzed by flow cytometry, we found that the surface expression of ERp57 and ERp72 is not different between WT and PDI-null platelets upon thrombin stimulation (Figure 1C). Additionally, we observed that WT and PDI-null platelets secrete similar levels of ERp57 and ERp72 in response to thrombin (data not shown). These results suggest that other thiol isomerases and ER chaperone proteins may compensate for the loss of intracellular PDI function.
Characterization of platelet-specific PDI–deficient mice. (A) Polymerase chain reaction (PCR) analysis of WT and PDI CKO mice with primers for floxed PDI (422 bp) and PF4-Cre (450 bp). (B) Lysates of neutrophils, endothelial cells, and platelets isolated from WT and PDI CKO mice were immunoblotted with indicated antibodies. Band density of PDI, ERp57, ERp72, and GRP78 in WT platelets (gray bars) is shown as 100%. White bars, PDI-null platelets. Data represent mean ± SD (n = 3-6 mice per group). **P < .01; ***P < .001 vs WT platelets after Student t test. (C) Flow cytometric analysis shows the surface expression of ERp57 and ERp72 on resting (dotted line) and thrombin-activated (black line) WT and PDI-null platelets. The gray histogram represents the fluorescence intensity of control IgG on thrombin-activated platelets. The geometric mean fluorescence intensity of antibodies was normalized to that of control IgG, and data are shown as a fold increase (mean ± SD, n = 3).
Characterization of platelet-specific PDI–deficient mice. (A) Polymerase chain reaction (PCR) analysis of WT and PDI CKO mice with primers for floxed PDI (422 bp) and PF4-Cre (450 bp). (B) Lysates of neutrophils, endothelial cells, and platelets isolated from WT and PDI CKO mice were immunoblotted with indicated antibodies. Band density of PDI, ERp57, ERp72, and GRP78 in WT platelets (gray bars) is shown as 100%. White bars, PDI-null platelets. Data represent mean ± SD (n = 3-6 mice per group). **P < .01; ***P < .001 vs WT platelets after Student t test. (C) Flow cytometric analysis shows the surface expression of ERp57 and ERp72 on resting (dotted line) and thrombin-activated (black line) WT and PDI-null platelets. The gray histogram represents the fluorescence intensity of control IgG on thrombin-activated platelets. The geometric mean fluorescence intensity of antibodies was normalized to that of control IgG, and data are shown as a fold increase (mean ± SD, n = 3).
Platelet PDI regulates agonist-induced platelet aggregation and ATP secretion, and the regulatory function is mediated by the isomerase activity of platelet surface PDI
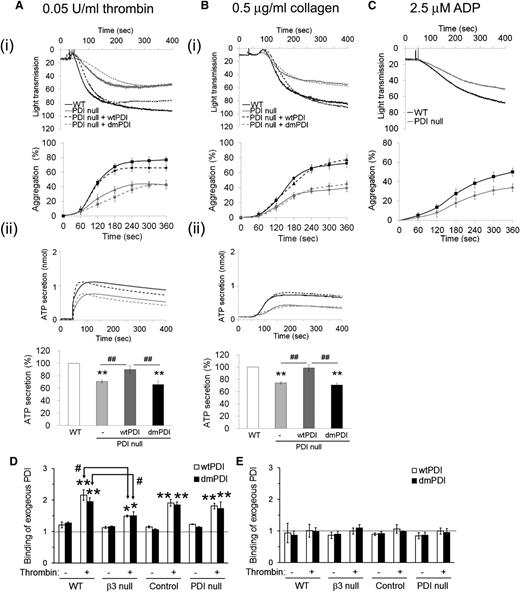
Previous studies showed that inhibition of platelet surface PDI reduces agonist-induced platelet aggregation.13,26 We observed that compared with WT platelets, PDI-null platelets exhibited significantly decreased aggregation and ATP secretion induced by low to intermediate concentrations of thrombin (<0.05 U/mL) and collagen (<0.5 μg/mL) (Figure 2A-B). Aggregation induced by ADP (2.5 μM) was also defective in PDI-null platelets in comparison with WT platelets (Figure 2C). The reduced aggregation and ATP secretion of PDI-null platelets were overcome at high concentrations of agonists (>0.1 U/mL thrombin, >2 μg/mL collagen, and >5 μM ADP) (supplemental Figure 3). Interestingly, recombinant wtPDI but not dmPDI (50 μg/mL) completely restored aggregation and ATP secretion of activated PDI-null platelets to the WT level (Figure 2A-B). When wtPDI was used at <20 μg/mL, the rescue effect was not observed (supplemental Figure 4). We also tested function-blocking monoclonal anti-PDI antibodies (BD34) in the aggregation assay. This antibody dose-dependently inhibited in vitro PDI activity in an insulin transhydrogenase assay and blocked thrombin-induced aggregation and ATP secretion of WT platelets (supplemental Figure 5A-B). In sharp contrast, the anti-PDI antibody did not affect thrombin-induced aggregation and ATP secretion of PDI-null platelets (supplemental Figure 5C). These results indicate that the isomerase activity of platelet surface but not intracellular PDI is required to regulate aggregation and ATP secretion of platelets.
Aggregation and ATP secretion of WT and PDI-null platelets and the rescue effect of recombinant PDI. Platelets isolated from WT (black line) and PDI CKO (gray line) mice were stimulated with 0.05 U/mL thrombin (A), 0.5 μg/mL collagen (B), and 2.5 μM ADP (C). (i) Platelet aggregation and quantitative graphs. (ii) ATP secretion. In some experiments, PDI-null platelets were pretreated with 50 μg/mL wtPDI (black dotted line) or dmPDI (gray dotted line) and activated with an agonist. Quantitative results of aggregation and ATP secretion are presented as mean ± SD (n = 4). **P < .01 vs WT platelets after ANOVA and Dunnett test; ##P < .01 vs PDI-null platelets treated with wtPDI after Student t test. (D,E) Mouse platelets were pretreated with His-tagged wtPDI or dmPDI and activated with or without thrombin in the presence of 1 mM EGTA under a stirring condition. Platelets were washed with HEPES-Tyrode buffer (D) or carbonate buffer (0.1 M Na2CO3, pH 9.0) (E). Binding of recombinant PDI was analyzed by flow cytometry using a Dylight 488–conjugated anti-polyHis antibody. PDI binding is shown as a fold increase by the ratio of the geometric mean intensity value of the anti-His antibody on PDI-treated vs untreated platelets (mean ± SD, n = 3). *P < .05; **P < .01 vs resting platelets; and #P < .05 vs activated WT platelets treated with wtPDI or dmPDI after Student t test.
Aggregation and ATP secretion of WT and PDI-null platelets and the rescue effect of recombinant PDI. Platelets isolated from WT (black line) and PDI CKO (gray line) mice were stimulated with 0.05 U/mL thrombin (A), 0.5 μg/mL collagen (B), and 2.5 μM ADP (C). (i) Platelet aggregation and quantitative graphs. (ii) ATP secretion. In some experiments, PDI-null platelets were pretreated with 50 μg/mL wtPDI (black dotted line) or dmPDI (gray dotted line) and activated with an agonist. Quantitative results of aggregation and ATP secretion are presented as mean ± SD (n = 4). **P < .01 vs WT platelets after ANOVA and Dunnett test; ##P < .01 vs PDI-null platelets treated with wtPDI after Student t test. (D,E) Mouse platelets were pretreated with His-tagged wtPDI or dmPDI and activated with or without thrombin in the presence of 1 mM EGTA under a stirring condition. Platelets were washed with HEPES-Tyrode buffer (D) or carbonate buffer (0.1 M Na2CO3, pH 9.0) (E). Binding of recombinant PDI was analyzed by flow cytometry using a Dylight 488–conjugated anti-polyHis antibody. PDI binding is shown as a fold increase by the ratio of the geometric mean intensity value of the anti-His antibody on PDI-treated vs untreated platelets (mean ± SD, n = 3). *P < .05; **P < .01 vs resting platelets; and #P < .05 vs activated WT platelets treated with wtPDI or dmPDI after Student t test.
To further examine how platelet PDI regulates ATP secretion, WT and PDI-null platelets were pretreated with EGTA to inhibit interaction of fibrinogen (FG) and other platelet-derived proteins with activated αIIbβ3 integrin and then stimulated with 0.05 U/mL thrombin.27 Both WT and PDI-null platelets did not aggregate but released an equal amount of ATP (supplemental Figure 6). Because ATP secretion is solely derived from agonist-induced platelet activation in the presence of EGTA, these results suggest that the defective ATP secretion in PDI-null platelets is derived from the reduced interaction of platelet-derived proteins such as FG with αIIbβ3 integrin.
We further determined whether the rescue effect of exogenous PDI is mediated by interaction of PDI with αIIbβ3 integrin. WT, β3-null, littermate control, and PDI-null platelets were treated with thrombin in the presence or absence of His-tagged wtPDI or dmPDI under a stirring condition. As analyzed by flow cytometry using a Dylight 488–conjugated anti-polyHis antibody, binding of both wtPDI and dmPDI to platelets was enhanced in response to thrombin (Figure 2D). However, compared with WT platelets, β3-null platelets exhibited significantly reduced PDI binding upon thrombin stimulation, showing that exogenous PDI binds to platelet β3 integrins. No difference was observed in PDI binding to control and PDI-null platelets. Notably, binding of recombinant PDI to platelets was completely dissociated by washing with carbonate buffer (0.1 M Na2CO3, pH 9.0) (Figure 2E), suggesting a charge-dependent interaction between PDI and surface molecules such as β3 integrins.28 Further, our finding that some PDI binding to β3-null platelets was retained in response to thrombin indicates potential interaction of PDI with other surface molecules.
Blocking anti-ERp57 antibodies further inhibit αIIbβ3 integrin activation, aggregation, and ATP secretion of PDI-null platelets without affecting platelet activation
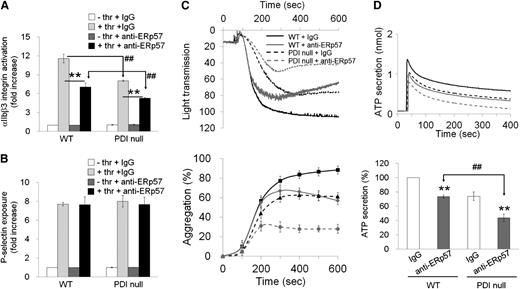
Recent reports showed that inhibition of surface ERp57, the closest homolog of PDI, with blocking antibodies significantly reduces platelet function and thrombus formation following vascular injury.20,29 To determine whether PDI and ERp57 play an overlapping or distinct role in platelet function, we assessed the contribution of surface ERp57 to PDI-null platelet function. In control experiments, αIIbβ3 integrin activation in PDI-null platelets was reduced by 30% to 40% in comparison with WT platelets in response to low and intermediate concentrations of thrombin (supplemental Figure 7A). Exogenously added wtPDI, but not dmPDI, rescued the defect in αIIbβ3 integrin activation on PDI-null platelets (supplemental Figure 7B). However, there was no difference in P-selectin exposure and Ca2+ mobilization between thrombin-activated WT and PDI-null platelets (supplemental Figure 7C-D). Inhibition of platelet surface ERp57 with blocking antibodies (50 μg/mL) further diminished αIIbβ3 integrin activation but not P-selectin exposure on PDI-null platelets (Figure 3A-B). Treatment of WT platelets with anti-ERp57 antibodies (50 μg/mL) significantly attenuated aggregation and ATP secretion induced by 0.05 U/mL thrombin (Figure 3C-D). However, the blocking antibody at 10 to 20 μg/mL did not show the inhibitory effect (supplemental Figure 8). Interestingly, compared with control IgG, the anti-ERp57 antibodies further reduced thrombin-induced aggregation and ATP secretion of PDI-null platelets (Figure 3C-D). These results suggest that PDI and ERp57 could play a distinct role in regulating αIIbβ3 integrin activation, platelet aggregation, and ATP secretion.
Inhibitory effect of surface ERp57 on αIIbβ3integrin activation, P-selectin exposure, aggregation, and ATP secretion of PDI-null platelets. Mouse platelets were pretreated with nonimmune sheep immunoglobulin G (IgG) or blocking anti-ERp57 antibodies (50 μg/mL) and stimulated with 0.05 U/mL thrombin (thr). (A,B) αIIbβ3 integrin activation and P-selectin exposure were analyzed by flow cytometry as described in “Materials and methods.” Binding of anti-activated αIIbβ3 (JON/A) and anti–P-selectin antibodies to platelets was calculated by the ratio of the geometric mean intensity value of antibodies to that of control IgG. Then, antibody binding to resting WT platelets treated with nonimmune sheep IgG was normalized as 1 (white bar in the left panel). Data represent mean ± SD (n = 3). **P < .01 vs control IgG; ##P < .01 vs WT platelets after Student t test. (C,D) Platelet aggregation and ATP secretion were measured as described in Figure 2 (mean ± SD, n = 3). **P < .01 vs control IgG; ##P < .01 vs WT platelets after Student t test.
Inhibitory effect of surface ERp57 on αIIbβ3integrin activation, P-selectin exposure, aggregation, and ATP secretion of PDI-null platelets. Mouse platelets were pretreated with nonimmune sheep immunoglobulin G (IgG) or blocking anti-ERp57 antibodies (50 μg/mL) and stimulated with 0.05 U/mL thrombin (thr). (A,B) αIIbβ3 integrin activation and P-selectin exposure were analyzed by flow cytometry as described in “Materials and methods.” Binding of anti-activated αIIbβ3 (JON/A) and anti–P-selectin antibodies to platelets was calculated by the ratio of the geometric mean intensity value of antibodies to that of control IgG. Then, antibody binding to resting WT platelets treated with nonimmune sheep IgG was normalized as 1 (white bar in the left panel). Data represent mean ± SD (n = 3). **P < .01 vs control IgG; ##P < .01 vs WT platelets after Student t test. (C,D) Platelet aggregation and ATP secretion were measured as described in Figure 2 (mean ± SD, n = 3). **P < .01 vs control IgG; ##P < .01 vs WT platelets after Student t test.
Platelet PDI does not affect β3–talin1 interaction, protein phosphorylation, or platelet spreading
It is known that agonist-induced signaling mediates talin binding to the cytoplasmic tail of the β3 subunit, which is critical for αIIbβ3 integrin activation.30 To examine whether platelet PDI regulates αIIbβ3 integrin activation through inside-out signaling, lysates of resting and activated WT and PDI-null platelets were immunoprecipitated with anti-αIIb antibodies, followed by immunoblotting with anti-β3 and anti-talin1 antibodies. We observed that interaction of the β3 subunit with talin1 increases upon thrombin stimulation, whereas the β3–talin1 interaction is not changed between activated WT and PDI-null platelets (Figure 4A). This result suggests that platelet PDI is unlikely to be involved in inside-out integrin signaling. We also observed similar patterns of protein phosphorylation between thrombin-activated WT and PDI-null platelets (Figure 4B).
Platelet PDI does not regulate the β3–talin1 interaction, protein phosphorylation, or platelet spreading on immobilized FG. (A) WT and PDI-null platelets (3 × 107 platelets in 0.3 mL) were treated with or without 0.05 U/mL thrombin (Thr) for 45 seconds in an aggregometer and lysed with ice-cold lysis buffer. Lysates were immunoprecipitated and immunoblotted as described in “Materials and methods.” Data represent mean ± SD (n = 4). (B) Protein phosphorylation levels were determined by immunoblotting with lysates of thrombin-activated WT and PDI-null (CKO) platelets using mouse IgG2b (mIgG2b) or an anti-phosphotyrosine antibody (4G10). The representative blot was obtained from 3 independent experiments. (C-E) Mouse platelets (8 × 106 platelets in 0.4 mL) were incubated on FG-coated surfaces for 2 h at 37°C in the presence of 0.025-0.05 U/mL thrombin. Adherent and spread platelets were stained with rhodamine-conjugated phalloidin. (C) Representative images. Bar = 10 μm. (D) Number of adherent (but not spread, black bars) and fully spread (white and gray bars) platelets. **P < .01 vs WT platelets (total number of adherent and spread platelets) after Student t test. (E) Platelet spreading was analyzed by the surface area, which was measured by the number of pixels divided by the number of platelets in the field. Data represent mean ± SD (n = 3).
Platelet PDI does not regulate the β3–talin1 interaction, protein phosphorylation, or platelet spreading on immobilized FG. (A) WT and PDI-null platelets (3 × 107 platelets in 0.3 mL) were treated with or without 0.05 U/mL thrombin (Thr) for 45 seconds in an aggregometer and lysed with ice-cold lysis buffer. Lysates were immunoprecipitated and immunoblotted as described in “Materials and methods.” Data represent mean ± SD (n = 4). (B) Protein phosphorylation levels were determined by immunoblotting with lysates of thrombin-activated WT and PDI-null (CKO) platelets using mouse IgG2b (mIgG2b) or an anti-phosphotyrosine antibody (4G10). The representative blot was obtained from 3 independent experiments. (C-E) Mouse platelets (8 × 106 platelets in 0.4 mL) were incubated on FG-coated surfaces for 2 h at 37°C in the presence of 0.025-0.05 U/mL thrombin. Adherent and spread platelets were stained with rhodamine-conjugated phalloidin. (C) Representative images. Bar = 10 μm. (D) Number of adherent (but not spread, black bars) and fully spread (white and gray bars) platelets. **P < .01 vs WT platelets (total number of adherent and spread platelets) after Student t test. (E) Platelet spreading was analyzed by the surface area, which was measured by the number of pixels divided by the number of platelets in the field. Data represent mean ± SD (n = 3).
Binding of platelet αIIbβ3 integrin to a ligand such as FG mediates stable adhesion and spreading of platelets. Because mouse platelets do not spread well on FG-coated surfaces without agonist stimulation,31 we used 0.025-0.05 U/mL thrombin in a spreading assay. We found that most (>90%) adherent WT and PDI-null platelets were spread on immobilized FG during the 2-hour incubation in the presence of 0.025-0.05 U/mL thrombin (Figure 4C-D). However, the number of adherent PDI-null platelets was reduced by 30% in comparison with WT platelets (Figure 4D). Because there could be differences in the kinetics of platelet adhesion over the 2-hour incubation, some PDI-null platelets attached to immobilized FG at later time points appeared to be dislodged by washing before full spreading. No difference was observed in lamellipodial actin assembly and surface coverage of WT and PDI-null platelets spread on FG-coated surfaces (Figure 4C,E). PDI-null platelets were observed to spread well after firm adhesion, consistent with the notion that other surface molecules may be involved in platelet spreading on immobilized FG.32 These results suggest that the role of platelet PDI is likely limited to initial platelet adhesion to FG-coated surfaces by regulating the activation and ligand binding activity of αIIbβ3 integrin.
Platelet PDI is important for thrombus formation on collagen-coated surfaces under arteriolar shear
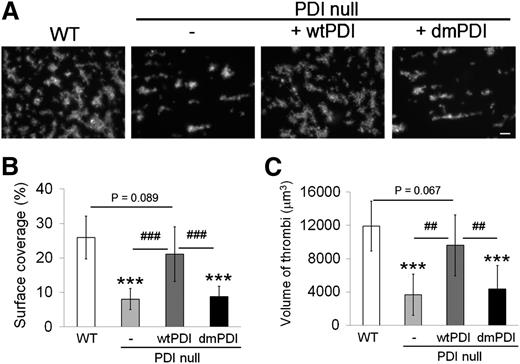
To assess the role of platelet PDI in in vitro thrombus formation, a flow chamber assay was performed under arteriolar shear (1000 s−1).24 Blood drawn from WT mice exhibited widespread coverage with densely packed platelet thrombi on collagen surfaces. In contrast, blood drawn from PDI CKO mice showed markedly reduced surface coverage (Figure 5A-B). Moreover, as determined by confocal microscopic analysis, the volume of PDI-null platelet thrombi significantly decreased in comparison with that of control WT thrombi (Figure 5C). When wtPDI was added to blood of PDI CKO mice, both surface coverage and volume of platelet thrombi were almost restored to the WT level. In marked contrast, no rescue effect was observed with dmPDI. These results indicate that the isomerase activity of platelet surface PDI is important for platelet adhesion and aggregation on collagen-coated surfaces under arteriolar shear. Both wtPDI and dmPDI, however, did not affect thrombus formation in blood of WT mice (data not shown), implying that endogenous PDI is sufficient for in vitro thrombus formation.
Platelet PDI is important for thrombus formation on collagen-coated surfaces under arteriolar shear. Blood drawn from WT and PDI CKO mice was perfused over collagen-coated surfaces at a wall shear rate of 1000 s−1 for 1 minute. In some experiments, wtPDI or dmPDI was added to blood drawn from PDI CKO mice and then blood was perfused through the chamber. Adherent thrombi were stained with rhodamine-conjugated phalloidin and analyzed as described in “Materials and methods.” (A) Representative images. Bar = 10 μm. (B,C) Surface coverage and thrombus volume were measured and presented as mean ± SD (n = 4). ***P < .001 vs WT platelets after ANOVA and Dunnett test; ##P < .01; and ###P < .001 vs PDI-null platelets treated with wtPDI after Student t test.
Platelet PDI is important for thrombus formation on collagen-coated surfaces under arteriolar shear. Blood drawn from WT and PDI CKO mice was perfused over collagen-coated surfaces at a wall shear rate of 1000 s−1 for 1 minute. In some experiments, wtPDI or dmPDI was added to blood drawn from PDI CKO mice and then blood was perfused through the chamber. Adherent thrombi were stained with rhodamine-conjugated phalloidin and analyzed as described in “Materials and methods.” (A) Representative images. Bar = 10 μm. (B,C) Surface coverage and thrombus volume were measured and presented as mean ± SD (n = 4). ***P < .001 vs WT platelets after ANOVA and Dunnett test; ##P < .01; and ###P < .001 vs PDI-null platelets treated with wtPDI after Student t test.
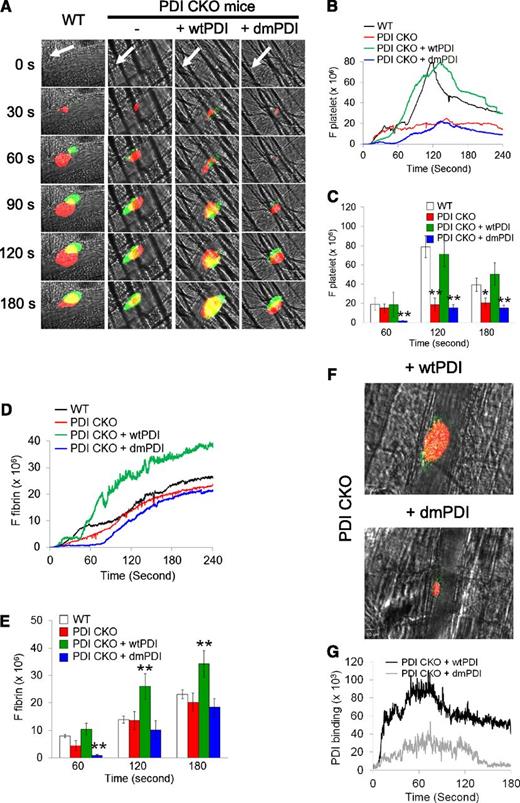
Platelet PDI is required for platelet accumulation but not initial adhesion and fibrin generation at the site of laser-induced arteriolar injury in live mice
Infusion of blocking anti-PDI antibodies into WT mice abolished platelet thrombus formation and fibrin generation following laser-induced arteriolar wall injury, indicating that PDI derived from intravascular cells plays a critical role during thrombus formation in vivo.15 Using fluorescence intravital microscopy in platelet-specific PDI–deficient mice, we determined the role of platelet PDI during arteriolar thrombus formation. Following laser-induced cremaster arteriolar wall injury,33 accumulating platelets and fibrin generation were visualized by infusion of Dylight 649–conjugated anti-mouse CD42c and Alexa 488–conjugated anti-fibrin antibodies, respectively. Both littermate WT control and platelet-specific PDI–deficient mice showed no significant difference in initial platelet adhesion until 60 s after laser-induced arteriolar injury (Figure 6A-C). However, platelet accumulation was significantly reduced at time of maximum activity (Tmax) in PDI CKO mice, compared with WT mice (supplemental Videos 1 and 2). Fibrin generation at the injury sites was slightly delayed until 90 s but similar at Tmax after vessel injury in the CKO mice in comparison with WT mice (Figure 6D-E). Consistent with the results in the in vitro assays, infusion of wtPDI (100 μg/mouse) into the PDI CKO mice rescued the defect of platelet accumulation to the WT level (Figure 6B-C; supplemental Video 3). Interestingly, fibrin generation was moderately but significantly enhanced from 1 minute after vascular injury in wtPDI-treated CKO mice, compared with WT or PDI CKO mice (Figure 6D-E). However, treatment of the CKO mice with dmPDI did not show any rescue effect and rather delayed platelet thrombus formation and fibrin generation at the injury site, compared with untreated PDI CKO mice (Figure 6B-E; supplemental Video 4). These results suggest that wtPDI may facilitate the function of endogenous PDI derived from endothelial cells and other blood cells, whereas dmPDI may perturb its function.
Platelet PDI plays a critical role in platelet accumulation but not initial adhesion and fibrin generation at the site of laser-induced arteriolar wall injury. Intravital microscopy was performed as described in “Materials and methods.” (A) Representative fluorescence images associated with platelets (red) and fibrin (green) are shown over the course of 180 seconds after vascular injury. White arrows show the direction of blood flow. (B-E) The median integrated fluorescence signals of anti-CD42c (F platelet) and anti-fibrin antibodies (F fibrin) were obtained from 28-30 thrombi in 3 WT or 6 CKO mice and is presented as a function of time. Little fluorescence signal was observed by fluorescently labeled control IgG (data not shown). (C,E) The fluorescence signal is shown at 60, 120, and 180 seconds after vascular injury. *P < .05; **P < .01 vs WT mice after Kruskal-Wallis test. (F) After infusion of wtPDI or dmPDI (100 μg), Dylight 649–conjugated rat anti-mouse CD42c and Dylight 488–conjugated anti-polyHis antibodies or mouse IgG1 were infused into PDI CKO mice. The representative images were obtained from 18-20 thrombi in 3 PDI CKO mice per group and are shown as Tmax for platelet thrombus formation. (G) The median integrated fluorescence signal of anti-polyHis antibodies (PDI binding) is presented as a function of time.
Platelet PDI plays a critical role in platelet accumulation but not initial adhesion and fibrin generation at the site of laser-induced arteriolar wall injury. Intravital microscopy was performed as described in “Materials and methods.” (A) Representative fluorescence images associated with platelets (red) and fibrin (green) are shown over the course of 180 seconds after vascular injury. White arrows show the direction of blood flow. (B-E) The median integrated fluorescence signals of anti-CD42c (F platelet) and anti-fibrin antibodies (F fibrin) were obtained from 28-30 thrombi in 3 WT or 6 CKO mice and is presented as a function of time. Little fluorescence signal was observed by fluorescently labeled control IgG (data not shown). (C,E) The fluorescence signal is shown at 60, 120, and 180 seconds after vascular injury. *P < .05; **P < .01 vs WT mice after Kruskal-Wallis test. (F) After infusion of wtPDI or dmPDI (100 μg), Dylight 649–conjugated rat anti-mouse CD42c and Dylight 488–conjugated anti-polyHis antibodies or mouse IgG1 were infused into PDI CKO mice. The representative images were obtained from 18-20 thrombi in 3 PDI CKO mice per group and are shown as Tmax for platelet thrombus formation. (G) The median integrated fluorescence signal of anti-polyHis antibodies (PDI binding) is presented as a function of time.
To further determine whether exogenously added PDI incorporates into the developing thrombus in vivo, binding of His-tagged PDI was visualized by infusion of a Dylight 488–conjugated anti-polyHis antibody. To quantify the fluorescence intensity of the anti-His antibody bound to recombinant PDI, the basal intensity of the antibody in the absence of recombinant PDI was subtracted. Both wtPDI and dmPDI were detected at the site of vascular injury and on the developing platelet thrombus (Figure 6F). We found that wtPDI binding increased as the size of the platelet thrombus grows, whereas dmPDI binding is relatively minimal, probably due to the small size of platelet thrombus and/or requirement for the active CGHC sequence for transient binding of PDI to surface molecules. (Figure 6G). Collectively, our results indicate that platelet PDI is required for stable thrombus growth but not initial platelet adhesion and fibrin generation at the site of laser-induced arteriolar injury and that the isomerase activity of extracellular PDI is crucial for the regulatory effect. This is consistent with previous studies showing that the initial source of PDI may be endothelial cells activated by laser injury.15,34
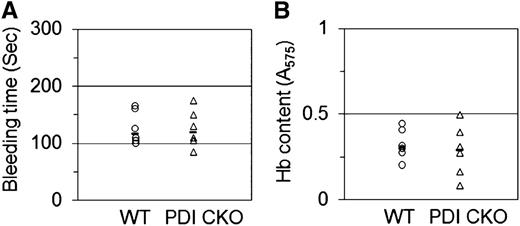
Platelet-specific PDI–null mice do not show significantly increased bleeding time and blood loss at the site of tail transection
Because previous studies showed that inhibition of extracellular PDI with blocking antibodies significantly prolongs bleeding time and increases blood loss at the site of tail transection in mice,15 we determined whether platelet-specific PDI deletion influences hemostatic function. Tail bleeding time was measured by the cessation of bleeding after tail amputation. Unlike inhibition of extracellular PDI,15 platelet-specific PDI–deficient mice did not display prolonged tail bleeding time in comparison with WT mice (117.5 ± 28.4 seconds [WT] vs 120.0 ± 32.8 seconds [CKO]) (Figure 7A). Blood collected from the site of amputation and quantified by hemoglobin content revealed no difference in blood loss between WT and PDI CKO mice (Figure 7B). These results, therefore, suggest that platelet PDI is not essential for hemostasis in mice.
Tail bleeding time and blood loss in platelet-specific PDI–deficient mice. Tails of WT (circle) and PDI CKO (triangle) mice were amputated, and bleeding time (A) was monitored as described in “Materials and methods.” (B) Blood loss during the bleeding time assay was determined by measuring the absorbance at 575 nm of hemoglobin (Hb). Horizontal bars represent the median of bleeding times and Hb content for each group of animals (n = 6).
Tail bleeding time and blood loss in platelet-specific PDI–deficient mice. Tails of WT (circle) and PDI CKO (triangle) mice were amputated, and bleeding time (A) was monitored as described in “Materials and methods.” (B) Blood loss during the bleeding time assay was determined by measuring the absorbance at 575 nm of hemoglobin (Hb). Horizontal bars represent the median of bleeding times and Hb content for each group of animals (n = 6).
Discussion
In the present study, we have demonstrated that platelet PDI regulates αIIbβ3 integrin activation without affecting P-selectin exposure, Ca2+ mobilization, protein phosphorylation, β3–talin1 interaction, or platelet spreading on immobilized FG. The isomerase activity of platelet surface PDI is essential for the regulatory effect. Unlike inhibition of extracellular PDI, which results in strong inhibition of platelet thrombus formation, fibrin generation, and hemostasis in live mice,15 platelet PDI CKO mice exhibited reduced thrombus growth but not defective initial platelet adhesion and fibrin generation at the site of laser-induced arteriolar injury. Importantly, platelet-specific PDI deletion did not impair hemostatic function at the site of tail transection. Thus, we provide the first evidence that platelet PDI is required for thrombus formation but is not essential for hemostasis in mice.
Studies with PDI gene deletion in yeast showed that PDI is necessary for cell viability,35 probably due to its critical function in the ER. However, as PDI has 21 family members in mammalian cells,25 it was thought that PDI gene deletion may result in a compensatory effect by other family members. Our results suggest that such compensation is likely limited to intracellular PDI function since the surface expression of ERp57 and ERp72 was not different between activated WT and PDI-null platelets and the expression of GRP78, an ER stress marker, was significantly upregulated in PDI-null platelets, probably due to the increased stress in the ER of megakaryocytes.36 PDI-null platelets exhibited defective aggregation and ATP secretion without a change in P-selectin exposure and Ca2+ mobilization. Interestingly, treatment of PDI-null platelets with anti-ERp57 antibodies additively inhibited αIIbβ3 integrin activation but not P-selectin exposure. Although the role of platelet ERp57 in α-granule secretion from activated human platelets is controversial,20,29 our studies with PDI-null platelets and anti-ERp57 antibodies clearly demonstrate that both PDI and ERp57 do not affect P-selectin exposure but regulate αIIbβ3 integrin activation. Although it is unclear why dense granule, but not α-granule, secretion is affected in PDI-null platelets, this result is probably due to the different kinetics of granule secretion. Following stimulation with low to intermediate concentrations of thrombin, most P-selectin may rapidly translocate to the plasma membrane during platelet activation, whereas ATP secretion could be rapid during cell activation and is likely also maintained after subsequent FG–αIIbβ3 interaction.
Extracellular PDI is important for regulating αIIbβ3 integrin–mediated platelet function.13,15,37 Recent studies have shown that PDI binds to β3 integrins in vitro and in vivo.16 Our results support the previous reports in several ways. First, we found that the isomerase activity of platelet surface PDI is important for regulating αIIbβ3 integrin activation. Further, given our finding that inhibition of surface ERp57 on PDI-null platelets additively diminishes integrin activation, surface PDI and other thiol isomerases such as ERp57 may regulate distinct stages of αIIbβ3 integrin activation and/or a different population of the integrin. Alternatively, each thiol isomerase may modulate the integrin function in a different manner; under certain redox conditions, PDI may isomerase sulfhydryl groups on the integrin, whereas ERp57 may oxidize or reduce the same or different sulfhydryl groups. This speculation is supported by previous studies showing that the isomerase activity of PDI is greater than that of ERp57.38 Nevertheless, we cannot rule out the possibility that there may be functional redundancy between PDI and ERp57 since some Cys residues in αIIbβ3 integrin might be oxidized or reduced by both PDI and ERp57 whereas others might be regulated by either PDI or ERp57.
Second, since platelet PDI regulated αIIbβ3 integrin activation without affecting P-selectin exposure, Ca2+ mobilization, protein phosphorylation, β3–talin1 interaction, and platelet spreading, our results imply that PDI secreted from activated platelets binds to and changes the structure of the extracellular domain of αIIbβ3 integrin. Thus, platelet surface but not intracellular PDI plays a regulatory role in integrin activation. This conclusion is further substantiated by the results from our rescue experiments in vitro and in vivo. It is of interest to note that binding of exogenous PDI to activated control and PDI-null platelets is equivalent, although some αIIbβ3 integrin and other surface molecules might be occupied by endogenous PDI released from activated control platelets. We found that one human platelet expresses ∼32 000 PDI molecules as determined by immunoblotting of recombinant human PDI and platelet lysates (supplemental Figure 9). Previous studies showed that ∼5% to 10% of total PDI is released from activated platelets.16 Therefore, endogenous PDI is likely to occupy only a small proportion of αIIbβ3 integrin (50 000-100 000 molecules per platelet) and other surface molecules. Therefore, the increased binding of exogenous PDI to control and PDI-null platelets would result from the limited amount of endogenous PDI released from platelets. Third, our intravital microscopic analysis demonstrated that PDI CKO mice exhibit a significant defect in αIIbβ3 integrin–mediated platelet accumulation at the site of laser-induced arteriolar injury. Nevertheless, it is unlikely that PDI interacts only with αIIbβ3 integrin. Platelet PDI could interact with other surface molecules such as α2β1 integrin and regulate their function.14 Indeed, this speculation is supported by our findings that exogenous PDI binding to β3-null platelets still increases upon platelet activation and that platelet adhesion to collagen-coated surfaces is impeded under arteriolar shear.
Consistent with our recent finding that PDI binds to activated neutrophil αMβ2 integrin in a charge-dependent manner,19 we observed that both wtPDI and dmPDI bind to αIIbβ3 integrin on activated platelets in the same fashion. Nevertheless, only wtPDI, but not dmPDI, rescued the defects in PDI-deficient platelet functions. These results suggest that initial binding of PDI to αIIbβ3 integrin is mediated by electrostatic interaction and then the active CGHC sites of PDI could facilitate thiol exchange on the integrin, presumably by transient disulfide bond formation between PDI and α/β subunits. Although thiol exchange on Cys residues on the integrin would be a reasonable explanation for PDI-regulated integrin activation,13,39 it will be challenging to directly identify the PDI-modified Cys residues on integrins since in vitro systems are unlikely to reproduce the redox potential under thrombotic conditions. PDI functions as a reductase or oxidoreductase dependent on the redox potential in a reaction environment.12 Therefore, manipulation of the in vitro condition could cause artifacts. Furthermore, PDI may not reduce or oxidize Cys residues in one direction but change the ratio of oxidation and reduction at the same Cys residues during integrin activation. Therefore, care must be taken to identify Cys residues that might undergo a different level of reduction or oxidation by PDI. Another complication is derived from the fact that the extracellular domains of the αIIb and β3 subunits contain 19 and 56 Cys residues, respectively. Because obtaining all Cys residues after digestion would be difficult, careful experimental conditions should be employed to detect the modified Cys residues.
It is reported that infusion of inhibitory anti-PDI antibodies into WT mice abrogates both platelet thrombus formation and fibrin generation following vascular injury, suggesting that extracellular PDI could be a target for a novel anti-thrombotic agent.15,40 Indeed, recent studies have shown that rutin, a natural flavonoid, selectively inhibits the activity of PDI over 4 thiol isomerases and has anti-thrombotic effects in vivo. However, we found that rutin markedly impedes aggregation and ATP secretion of PDI-null platelets (supplemental Figure 10), indicating that the flavonoid has an additional effect other than PDI inhibition. In addition to platelets, activated intravascular cells including endothelial cells and leukocytes could release PDI following vascular injury.15,19 Our intravital microscopic analysis with platelet-specific PDI-deficient mice clearly demonstrated that platelet PDI is mainly involved in platelet accumulation but not initial adhesion and fibrin generation at the site of laser-induced arteriolar injury. Initiation of platelet adhesion and fibrin generation following laser-induced arteriolar injury could be regulated by PDI derived from activated endothelial cells. Notably, infusion of wtPDI into PDI CKO mice rescued the defect in platelet accumulation and even moderately enhanced fibrin generation at the site of arteriolar injury. In addition to the importance of platelet PDI activity for platelet accumulation,15 our results suggest that exogenous PDI could bind to surface molecules on activated endothelial cells and may facilitate fibrin generation. It is interesting to note that platelet-specific PDI deletion does not significantly increase bleeding time and blood loss at the site of tail transection, probably because of both small platelet thrombus formation and normal fibrin generation in the CKO mice. Noting that tail bleeding time may not be a reliable assay to test platelet contribution to hemostatic function, we also observed no increased bleeding from the surgery site and cremaster muscle in PDI CKO mice in comparison with WT mice during in vivo intravital microscopic studies. Inhibition of extracellular PDI or ERp57 abolishes thrombus formation and significantly prolongs tail bleeding time in mice.15,29 Although we cannot completely rule out the possibility that the minimal amount (3%) of platelet PDI may play a role in initial platelet adhesion and hemostasis, thiol isomerases derived from other intravascular cells such as endothelial cells are likely important for hemostatic function in mice. Taken together, our results provide important evidence that pharmacological inhibitors for thiol isomerases should be carefully evaluated for the risk–benefit ratio. Future studies of mice deficient in endothelial cell–specific PDI will be extremely helpful for understanding how intravascular PDI plays a role during thrombosis and hemostasis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Hartmut Weiler for providing the monoclonal anti-fibrin antibody (59D8), Dr Deane F. Mosher for having read the manuscript and his thoughtful suggestions, and Mr Ronald McKinney for technical support for isolation of cardiac endothelial cells. J.M.G. is a visiting professor at King Saud University, Riyadh, Saudi Arabia.
This work was supported in part by grants from National Institutes of Health, National Heart, Lung, and Blood Institute (P30HL101302 and R01HL109439 to J.C.), American Heart Association (SDG 5270005 to J.C.), and the University of Illinois at Chicago Center for Clinical and Translational Science award (CCTS0413 to J.C.).
Authorship
Contribution: K.K. designed and performed research, collected and analyzed data, and wrote the manuscript; E.H. performed research, analyzed data, and wrote the manuscript; J.L. performed research; L.-M.H., P.S., R.G.S., and M.U.-F. provided important reagents; J.M.G. provided important reagents and revised the manuscript; and J.C. initiated, designed, and performed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jaehyung Cho, 835 S Wolcott Ave, E403 (5095 CoMRB), Chicago, IL 60612; e-mail: thromres@uic.edu.