To the editor:
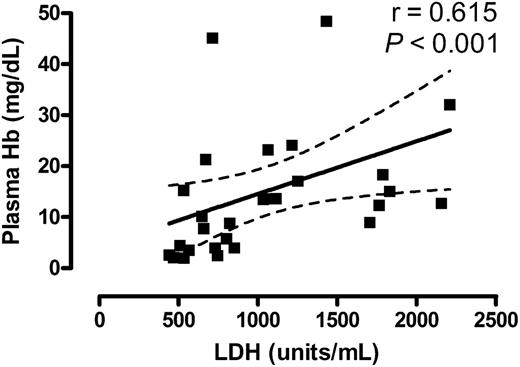
Dr Ballas has provided a thoughtful perspective on the meaning of elevated serum lactate dehydrogenase (LDH) in sickle cell disease.1 He is clearly correct that serum LDH is generally high at steady state in sickle cell disease and comes from multiple sources, representing damage to cells from several different organs, but this is not the entire story. There are several lines of published data that address the questions he raises concerning the relationship of serum LDH to lysis of red cells. Our publication in Blood 7 years ago clearly documented the LDH isoenzyme data he requests. It showed at steady state an average of 71% of total LDH was derived from a combination of LD1 and LD2, reflecting disproportionate elevation of isoenzymes that are consistent with red cell origin.2 In fact, 96% of the specimens had LD1 levels above the expected range; isoforms of liver, muscle, lymphocytes, and platelets were underrepresented in total LDH.2 We also showed that in catheter-drawn specimens processed at bedside to decrease artifactual hemolysis, serum LDH correlated with plasma hemoglobin, a well-accepted marker of intravascular hemolysis (r = 0.73, P < .01).2 Although Neely et al carefully measured serum LDH and plasma hemoglobin released during hemolysis and did not assert their correlation,3 current analysis of their original data supports precisely such an association. Using the open-source digitizing software Enguage4 to convert Neely et al’s Figure 2 to digital values for LDH and free hemoglobin, statistical analysis of log-transformed data in GraphPad Prism 5.0 software shows a statistically significant Pearson correlation (r = 0.615, P < .001; Figure 1). Linear regression analysis of log-transformed data suggests that variations in plasma hemoglobin account for approximately 38% of the variation in LDH (P = .0004).
Correlation of serum LDH with plasma hemoglobin. Plot of data points derived from Neely and colleagues,3 with the solid line representing linear regression and the dashed lines indicating 95% confidence interval. Significance was calculated by Pearson correlation analysis of log-transformed data.
Correlation of serum LDH with plasma hemoglobin. Plot of data points derived from Neely and colleagues,3 with the solid line representing linear regression and the dashed lines indicating 95% confidence interval. Significance was calculated by Pearson correlation analysis of log-transformed data.
LDH also strongly correlates with another product of hemolyzing red cells, erythrocyte-derived microparticles, in a study from Amsterdam (r = 0.59, P < .001).5 A parallel result emerges from our analysis from a transcontinental multicenter trial in which LDH (adjusted for different LDH assays by site) from untransfused sickle cell anemia patients in the highest quartile of hemolytic component correlates with the count of erythrocyte-derived microparticles (r = 0.36, P = .006, n = 57) and other hemolytic markers.6
Our conclusion from these publications and our own data is that at steady state in adults with sickle cell disease, hemolysis contributes significantly but nonexclusively to the serum LDH value. During vaso-occlusive crisis, LDH rises at least in part due to lysis of red cells, as shown by Dr Ballas in definitive chromium radiolabeling red cell survival studies7 and by several others in which LDH rises as hemoglobin levels fall,7-10 undoubtedly accompanied by variably increased LDH due to lysis of cells from other organs. We all should freely acknowledge the diversity of contributory organ LDH sources and prominent variability during transition from steady state to vaso-occlusive crisis, but we should not overlook the disproportionate contribution of red cells during steady state and that serum LDH in part represents intravascular hemolysis and release of plasma hemoglobin.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gregory J. Kato, National Institutes of Health, 9000 Rockville Pike, MSC 1476, Building 10-CRC, Room 5-5140, Bethesda, MD 20892-1476; e-mail gkato@nhlbi.nih.gov.