In this issue of Blood, Garçon et al describe successful derivation of induced pluripotent stem cells (iPSs) from fibroblasts of Diamond Blackfan anemia (DBA) patients with 2 distinct ribosomal defects. Using these cells, the authors showed that they not only exhibit defective erythropoiesis but also globally impaired hematopoiesis affecting multipotent progenitors.1
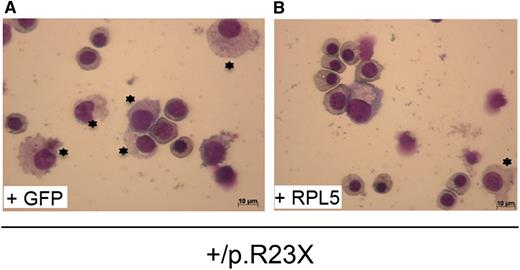
The defect in erythroid progenitors from RPL5+/R23X iPSs generated from DBA affected fibroblasts (A), where most of the cells are myeloid cells, and the rescue by the wild RPL5 transgene, where most of the cells are erythroid progenitors (B). See the complete Figure 7 in the article by Garçon et al that begins on page 912.
The defect in erythroid progenitors from RPL5+/R23X iPSs generated from DBA affected fibroblasts (A), where most of the cells are myeloid cells, and the rescue by the wild RPL5 transgene, where most of the cells are erythroid progenitors (B). See the complete Figure 7 in the article by Garçon et al that begins on page 912.
DBA is the first human ribosomopathy described. Mutations (among them large deletions in genes encoding a number of ribosomal proteins that include RPS19, RPL5, RPL11, RPS10, RPS26, RPS24, RPL35a, RPS17, RPS7, RPL36) have been identified in 70% of affected DBA patients.2 Haploinsufficiency of these ribosomal proteins results in distinct ribosomal RNA maturation defects, which in turn generates a nucleolar stress leading to the activation of p53 pathway. Indeed, activation of p53 has previously been shown to result in increased apoptosis and cell-cycle arrest in G0/G1 of erythroid cells in DBA accounting for the aregenerative anemia in the peripheral blood of patients.3,4 It should, however, be noted that pathways other than p53 activation could also play a role in defects in DBA.5
A major challenge in defining the mechanistic basis for hematopoietic defects in DBA has been the limited access to primary hematopoietic stem cells of DBA individuals. Previous attempts to generate iPS cells using fibroblasts of DBA individuals have met with little success possibly due to activation of p53. The successful derivation of 2 clones of IPSs, 1 with mutant RPS19 and another with mutant RPL5 from DBA affected DBA patients by Garçon et al, is thus a major accomplishment and has the potential to open new avenues of research toward our understanding of this hematologic disorder.
The prevailing concept is that the hematopoietic defect in DBA affects primarily erythroid lineage and that the erythroid defect occurs at the progenitor stages between burst-forming unit-erythroid erythropoietin (EPO)-independent and colony-forming unit-erythroid EPO-dependent stages. For this reason, significant efforts in DBA research were focused on identifying causative mutations in erythroid genes such as EPO and EPO-R, SCF and c-kit, GATA-1. Thus, it came as a big surprise in 1999 when mutations in the gene-encoding ribosomal protein, RPS19, were first identified in association with DBA. In fact, there was considerable skepticism about the relevance of this finding because it was difficult to explain how mutations in a gene encoding a ribosomal protein result predominantly in anemia. Now that mutations in a large number of ribosomal proteins have been documented to account for DBA phenotype in >70% of cases, it is clear that DBA is indeed a ribosomopathy.
What are the implications of the reported findings? The findings of Garçon et al clearly show that haploinsufficiency of RPS19 and RPL5 results in defective erythroid differentiation of hematopoietic stem cells derived from iPS cells carrying ribosomal protein mutations (see figure). Importantly, rescue of the phenotype by expression of wild-type RPS19 and RPL5 in iPS cells validates that these mutations are indeed causative of defective erythropoiesis in DBA. Furthermore, their findings that ribosomal protein defects are pleotropic, affecting multiple hematopoietic lineages, is indeed exciting and implies we have to broaden our views on the role of ribosome biogenesis on all aspects of hematopoiesis. It is important to note that such pleotropic effects were suggested by the earlier work of Giri et al,6 Santucci et al,7 and Casadevall et al8 but these efforts have not received the recognition that they deserve.
The present findings also challenge the current perception that primitive and fetal erythropoiesis are not affected in DBA because most of the cases are diagnosed during infancy with only 16% of DBA cases diagnosed at birth and in the neonatal period. It is very likely that, in some instances, the DBA phenotype is expressed during fetal development as indicated by the recent identification of a mutation in the RPS19 gene in a case of hydrops fetalis9 along with other cases of hydrops fetalis in DBA families.
The present study by Garçon and colleagues represents the first step in what should prove to be a long and productive journey toward a comprehensive understanding of the role of ribosomes in normal and disordered hematopoiesis and in evaluating new therapeutic options for the treatment of DBA.
Conflict-of-interest disclosure: The author declares no competing financial interests.