Key Points
Retained FcRn binding of an IgG3 antibody devoid of FcgR and C1q binding, cellular cytotoxicity and complement activation.
Inhibition of pathogenic polyclonal anti-D in antibody-dependent cellular toxicity by a hinge region deleted anti-D IgG3 antibody with efficient transplacental transport capacity.
Abstract
The neonatal Fc receptor (FcRn) directs the transfer of maternal immunoglobulin G (IgG) antibodies across the placenta and thus provides the fetus and newborn with passive protective humoral immunity. Pathogenic maternal IgG antibodies will also be delivered via the placenta and can cause alloimmunity, which may be lethal. A novel strategy to control pathogenic antibodies would be administration of a nondestructive IgG antibody blocking antigen binding while retaining binding to FcRn. We report on 2 human IgG3 antibodies with a hinge deletion and a C131S point mutation (IgG3ΔHinge) that eliminate complement activation and binding to all classical Fcγ receptors (FcγRs) and to C1q while binding to FcRn is retained. Additionally, 1 of the antibodies has a single point mutation in the Fc (R435H) at the binding site for FcRn (IgG3ΔHinge:R435H). We compared transplacental transport with wild-type IgG1 and IgG3, and found transport across trophoblast-derived BeWo cells and ex vivo placenta perfusions with hierarchies as follows: IgG3ΔHinge:R435H>wild-type IgG1≥IgG3ΔHinge and IgG3ΔHinge:R435H=wild-type IgG1=wild-type IgG3>>>IgG3ΔHinge, respectively. Collectively, IgG3ΔHinge:R435H was transported efficiently from the maternal to the fetal placental compartment. Thus, IgG3ΔHinge:R435H may be a good candidate for transplacental delivery of a nondestructive antibody to the fetus to combat pathogenic antibodies.
Introduction
During pregnancy, placental villi are submerged in maternal blood with large amounts of immunoglobulin G (IgG). In the third trimester, IgG is efficiently transported across the placenta from the mother and fetal serum IgG concentration approaches or exceeds that of the mother. It is now generally accepted that a cellular receptor named the neonatal Fc receptor (FcRn) is pivotal for maternofetal IgG transport, and thus provides the fetus and newborn with humoral immune protection until the infant starts producing its own IgG.1-4 FcRn-IgG complex formation also rescues IgG from degradation via a cellular recycling pathway that takes place in hematopoietic cells and vascular endothelial cells that coat the blood vessels.5-8 The interaction between FcRn and IgG exhibits strict pH dependency, with binding at pH 6 and release and no binding at neutral pH.6,8,9 FcRn primarily resides within acidified endosomal compartments and there encounters and binds the IgG that is continuously taken up by pinocytosis from the blood. The FcRn-IgG complex is transported to the cell surface where exposure to the neutral pH of the blood triggers rapid release of IgG back to the circulation. The pH dependence is explained by the involvement of histidine residues located at amino acid positions 310 and 435 at the junction between the CH2 and CH3 domains of the IgG.6,8,9 When protonated at acidic pH, histidine residues interact with negatively charged residues on FcRn. All human IgG subclasses have histidines at these 2 positions, except IgG3 which has an arginine at position 435 (R435). This single amino acid difference has recently been shown to explain why IgG3 has a threefold shorter half-life as compared with the other 3 subclasses.10 Furthermore, mutation of H435 to alanine in IgG1 has been demonstrated to result in loss of binding to FcRn, which gives a dramatic drop in serum half-life of human IgG1 injected into mice, and a reduced transfer across an ex vivo human placental model.1,8 These findings show that binding to FcRn is a prerequisite for extended serum half-life and for transport of IgG antibodies across the maternofetal barrier.
However, pathogenic maternal antibodies with specificity for fetal antigens are transported and regulated in the same way and can cause fetal alloimmune disease that may prove lethal to the fetus and the newborn. Binding of pathogenic antibody to its cognate antigen initiates elimination and destruction of the target cell to which it is attached. One example is fetal and neonatal alloimmune thrombocytopenia (FNAIT), a severe bleeding disorder in which maternal antibodies cross the placenta and sensitize fetal platelets leading to their elimination.11 Another is hemolytic disease of the fetus and newborn (HDFN) in which maternal antibodies sensitize red blood cells (RBCs) in the fetal circulation and subsequently induce lysis that causes fetal anemia.12 The main treatment of FNAIT is intravenous Ig (IVIG) or steroid, or a combination,13 and for HDFN intrauterine transfusion and IVIG has been used.14 Also, plasmapheresis has been used clinically in both diseases to reduce fetal antibody-mediated morbidity by removing pathogenic antibody. A murine FNAIT model showed that both IVIG and anti-FcRn antibodies ameliorate the effect of pathogenic antibodies and reduce the transplacental transport of pathogenic antibodies.15 Similarly, the common effect of IVIG and plasmapheresis is a reduction in the total amount of pathogenic antibody in the mother as well as in the fetus. Importantly, such strategies may also inhibit transfer of immune-protective maternal IgG and ultimately lead to reduced fetal and neonatal humoral immunity. This may cause an increased risk for infections during pregnancy as well as during the first weeks after birth.
Therefore, more specific and effective antenatal therapies are desirable. An attractive, alternative strategy to control fetal disease caused by pathogenic maternal IgG would be to administer to the mother a nondestructive IgG antibody sharing the specificity of the pathogenic antibody and retaining the ability to be transported across the placenta. Notably, on the molecular level, the IgG given must lack the ability to activate complement and not bind classical Fcγ receptors (FcγRs) that induce effector functions, whereas binding to FcRn must be preserved.
We previously investigated the functional properties of a recombinant anti-D IgG3 antibody with a hinge region deletion (IgG3ΔHinge) that resembles the hinge-deleted myeloma proteins Dob16 and Mcg.17 IgG3ΔHinge is inactive in both complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity as measured in functional assays.18,19 Here, we investigated the binding and transport properties of the Fc-engineered IgG3 antibodies, IgG3ΔHinge and IgG3ΔHinge:R435H, by comparison with wild-type IgG1 and IgG3. Specifically, we investigated binding to all classical FcγRs, FcRn, and C1q, and measured transcytosis in a BeWo cell transport assay20 and transcellular transport in an ex vivo placental transport model.21 We demonstrate that IgG3ΔHinge:R435H antibodies are efficiently transplacentally delivered from the maternal to the fetal compartment of ex vivo placenta perfusions at a rate similar to that of human IgG1 wild type and, by comparing wild-type IgG3 with IgG3ΔHinge, find indications of an enhancing effect of the hinge region.
Methods
Construction and production of antibodies
The Cγ chain of wild-type control IgG1 has allotype G1m(a,z) whereas IgG3 has G3m(b). IgG3ΔHinge was previously described and termed m0/C131S18 and HM5.19 Similarly, IgG3ΔHinge:R435H was termed HM5R435H.19 The anti-D antibodies, α-D (IgG1, IgG3ΔHinge, and IgG3ΔHinge:R435H), were termed IgG1+GAN, HM5+GAN, and HM5R435H+GAN, respectively,19,22 and the anti-malaria antibodies, α-MSP-3 (IgG1 and IgG3), were termed IgG1+RAM1 and IgG3+RAM1.23 All antibodies were produced and purified essentially as previously described.19,22,23
Receptor-binding studies
Microtiter plates were coated with equal amounts of antibodies (supplemental Figure 2, available on the Blood website) and incubated with glutathione S-transferase (GST)–fused recombinant soluble forms of human FcγRs (hFcγRI, hFcγRIIa, hFcγRIIb, hFcγRIIIa, and hFcγRIIIb).24,25 All receptors were diluted in 1× phosphate-buffered saline (PBS)/Tween 20 pH 7.4 and added to the wells at a concentration of 1 µg/mL except for shFcγRI, which was added at a concentration of 0.25 µg/mL. Bound receptors were detected using a horseradish peroxidase–conjugated anti-GST antibody produced in goats (GE Healthcare). In addition, GST-tagged soluble human FcRn (hFcRn-GST)26 was added (1 µg/mL), and the enzyme-linked immunosorbent assay (ELISA) was performed at either pH 7.4 or pH 6.0. Bound receptor was detected with horseradish peroxidase–conjugated anti-GST, followed by addition of 100 μL of 3,3′,5,5′-tetramethylbenzidine substrate (Calbiochem). The absorbance was measured at 620 nm using a Sunrise TECAN spectrophotometer.
Alkaline phosphatase–conjugated protein A from Staphylococcus aureus (Sigma-Aldrich) was diluted in 1× PBS/Tween 20 pH 7.4 and added to wells coated with titrated amounts of the antibodies (6.0-0.09 μg/mL). After incubation for 1 hour at room temperature, the wells were washed 4 times with 1× PBS/Tween 20 pH 7.4, and detection was done using alkaline phosphatase substrate (Sigma-Aldrich) followed by measurement of absorbance at 405 nm.
BeWo cell monolayer transfer model
The BeWo clone b30 was provided by Dr Margaret Saunders (Bristol Haematology and Oncology Centre, Bristol, UK) with permission from Dr Alan Schwartz (Washington University, St. Louis, MO). The cell-culture protocol previously described by Bode et al,27 was used, and the BeWo monolayer transport model was adapted from Poulsen et al.28 More details are provided in supplemental Methods.
Human placental transfer model
The ex vivo human placental perfusion model has previously been described.21,29 Placentas from uncomplicated pregnancies resulting in vaginal birth or caesarean section were donated from women giving birth at the Copenhagen University Hospital (Rigshospitalet). To minimize variation, mothers who smoked or had diabetes or other pregnancy complications were excluded from the study. Only term placentas were included. The project was approved by the ethical committees in the Communities of Copenhagen and Frederiksberg (KF 01-145/03 + KF (11) 260063) and the Danish Data Protection Agency. Informed consent was obtained in accordance with the Declaration of Helsinki. More details are provided in supplemental Methods.
SOL-ELISA for specific quantification of anti-D antibodies
Statistics
All statistical analyses were performed with SAS Statistical Software, version 9.2. Correlation between endogenous antibody concentration and recombinant antibody fetomaternal ratio was tested with Spearman rank correlation test. To distinguish the time point at which the fetal concentration of the recombinant antibody in the placental perfusion samples was different from zero, the fetal recombinant antibody concentration was tested at all time points using a 1-sample t test. The same test was also performed for the basolateral concentration in the BeWo model. For statistical purposes, the background or zero concentration in the perfusion model was defined as the average concentrations on the fetal side of α-MSP-3 (IgG1), α-D (IgG1), α-MSP-3 (IgG3), IgG3ΔHinge, and IgG3ΔHinge:R435H from zero to 60 minutes. The fetomaternal ratios of α-D (IgG1), IgG3ΔHinge, and IgG3ΔHinge:R435H were compared with the values for the control antibody studied in the same perfusion at each time point using a paired 2-sample t test for the means. In the BeWo model, the antibodies were compared using an unpaired 2-sample t test. The differences were defined to be statistically significant when P < .05. The data are presented as the mean ± SD, unless otherwise stated.
Results
Design of engineered human IgG antibodies
Wild-type recombinant antibodies contained the heavy chains from either human IgG1 or IgG3 which were paired with human κ light chains, and harbored specificity for the RhD antigen on human RBCs, α-D (IgG1, IgG3ΔHinge, and IgG3ΔHinge:R435H),18,19,22 or the malaria antigen MSP-3 from Plasmodium falciparum, α-MSP-3 (IgG1 and IgG3).23 The human IgG3ΔHinge antibodies have all 4 hinge region exons deleted, and lack a disulfide bridge between their light and heavy chains due to a cysteine to serine mutation at amino acid position 131 of the CH1 domains (C131S). This allows the 2 light chains to form an interchain disulfide bridge through their C-terminal cysteines. The resulting recombinant IgG3 mimics the myeloma proteins Dob and Mcg, and like these, which are derived from IgG1, it has a Lys in position 133.16-18 In addition, we introduced a single mutation within the CH3 domains of IgG3ΔHinge by mutating an arginine at position 435 to a histidine (R435H), which makes it more IgG1-like at the FcRn-binding site, and we designated the resulting antibody IgG3ΔHinge:R435H. The amino acid sequences of the heavy chain C region from wild-type IgG3, IgG3ΔHinge, and IgG3ΔHinge:R435H are aligned in supplemental Figure 1. Schematic illustrations of the different IgG formats constructed are shown in Figure 1.
Schematic illustration of the engineered antibodies. (A-B) Schematic illustrations of the domain architecture of wild-type IgG1 and IgG3. (C-D) The 2 engineered IgG3 antibodies, IgG3ΔHinge and IgG3ΔHinge:R435H, both have a deletion of the hinge region and a mutation within the CH1 domain (C131S), making them unable to activate complement and FcγR-mediated functions, which are the 2 main effector systems leading to cellular destruction. Compared with IgG3ΔHinge, the IgG3ΔHinge:R435H antibody has a mutation in the CH3 domain of the Fc where an arginine at position 435 is exchanged to a histidine, making IgG3ΔHinge:R435H identical to IgG1 in this position.
Schematic illustration of the engineered antibodies. (A-B) Schematic illustrations of the domain architecture of wild-type IgG1 and IgG3. (C-D) The 2 engineered IgG3 antibodies, IgG3ΔHinge and IgG3ΔHinge:R435H, both have a deletion of the hinge region and a mutation within the CH1 domain (C131S), making them unable to activate complement and FcγR-mediated functions, which are the 2 main effector systems leading to cellular destruction. Compared with IgG3ΔHinge, the IgG3ΔHinge:R435H antibody has a mutation in the CH3 domain of the Fc where an arginine at position 435 is exchanged to a histidine, making IgG3ΔHinge:R435H identical to IgG1 in this position.
Binding to classical human FcγRs and C1q, complement activation, and inhibition of ADCC
To investigate the FcγR-binding properties of the constructed antibodies, they were screened using ELISA for binding to all classical human FcγRs using recombinant GST-tagged soluble receptor (hFcRn, hFcγRI, hFcγRIIa, hFcγRIIb, hFcγRIIIa, and hFcγRIIIb). Prior to receptor binding, the amounts of antibodies were carefully normalized using a polyclonal anti-human IgG Fc preparation that showed equal input amounts (supplemental Figure 2). Then, equal amounts of the antibodies were coated in ELISA wells followed by adding hFcγRs, and bound receptors were visualized using an enzyme-conjugated anti-GST antibody (Figure 2). While wild-type IgG1 and IgG3 were shown to bind strongly to all the hFcγRs, the engineered IgG3 variants with hinge-region deletions (IgG3ΔHinge and IgG3ΔHinge:R435H) did not bind. In addition, the R435H mutation did not have any impact on binding. This is in line with previous data showing that this mutation does not restore effector functions.18,19 In addition, screening for binding toward human C1q showed that neither IgG3ΔHinge nor IgG3ΔHinge:R435H binds (supplemental Figure 3).
Binding of wild-type and engineered IgGs to classical human FcγRs. ELISA screening showing binding of titrated amounts of wild-type IgG1, IgG3, and engineered IgG3 variants to (A) hFcγRI, (B) hFcγRIIaH131, (C) hFcγRIIb, (D) hFcγRIIIa, and (E) hFcγRIIIb. The hinge-deleted antibodies IgG3ΔHinge and IgG3ΔHinge:R435H do not bind to any of the receptors; n = 3. All data are presented as mean ± SD.
Binding of wild-type and engineered IgGs to classical human FcγRs. ELISA screening showing binding of titrated amounts of wild-type IgG1, IgG3, and engineered IgG3 variants to (A) hFcγRI, (B) hFcγRIIaH131, (C) hFcγRIIb, (D) hFcγRIIIa, and (E) hFcγRIIIb. The hinge-deleted antibodies IgG3ΔHinge and IgG3ΔHinge:R435H do not bind to any of the receptors; n = 3. All data are presented as mean ± SD.
We also investigated complement activation by IgG3ΔHinge and IgG3ΔHinge:R435H and found no production of C1q, C3c, or C5 (supplemental Figures 4-6).18 This was corroborated by demonstrating that neither IgG3ΔHinge nor IgG3ΔHinge:R435H lysed erythrocytes in antibody-dependent complement-mediated lysis (supplemental Figure 7). Antibody-dependent cellular cytotoxicity (ADCC) demonstrated absence of lysis by IgG3ΔHinge and IgG3ΔHinge:R435H and indeed both molecules inhibited lysis by pathogenic polyclonal anti-D (supplemental Figures 8-9).19
pH-dependent binding to hFcRn
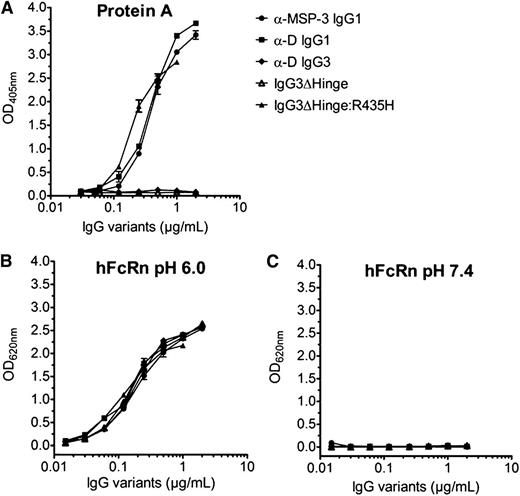
To investigate hFcRn-binding activities of the recombinant IgG variants, pH-dependent binding to a GST-tagged form of the receptor was performed using the ELISA procedures described in “Methods.” All IgGs, including the hinge-engineered IgG3 variants, were shown to bind hFcRn in a strictly pH-dependent manner (ie, strong binding at pH 6.0 with only negligible differences, and no detectable binding at neutral pH [Figure 3]). Introduction of the R435H point mutation into IgG3 was verified by gain of binding to protein A from S aureus, as H435 is required for protein A binding to human IgG1.10,32 The IgG3ΔHinge:R435H variant showed strong binding to hFcRn at acidic pH.
Binding of wild-type and engineered IgGs to protein A and hFcRn. ELISA screening showing binding of titrated amounts of wild-type IgG1, IgG3, and engineered IgG3 variants to (A) protein A from S aureus, and (B) hFcRn at pH 6.0 and (C) hFcRn at pH 7.4. Wild-type IgG3 and IgG3ΔHinge do not bind to protein A, which confirms that IgG3 and IgG3ΔHinge lack the amino acid residue histidine in position 435. All antibodies bind to hFcRn at pH 6.0, but not at pH 7.4; n = 3. All data are presented as mean ± SD.
Binding of wild-type and engineered IgGs to protein A and hFcRn. ELISA screening showing binding of titrated amounts of wild-type IgG1, IgG3, and engineered IgG3 variants to (A) protein A from S aureus, and (B) hFcRn at pH 6.0 and (C) hFcRn at pH 7.4. Wild-type IgG3 and IgG3ΔHinge do not bind to protein A, which confirms that IgG3 and IgG3ΔHinge lack the amino acid residue histidine in position 435. All antibodies bind to hFcRn at pH 6.0, but not at pH 7.4; n = 3. All data are presented as mean ± SD.
Transcytosis of IgG across BeWo cells
To address whether or not IgG3ΔHinge and IgG3ΔHinge:R435H were transported across a polarized cellular layer, the human trophoblast-derived BeWo cell line was used. This cell line has previously been demonstrated to express hFcRn and mediate IgG transport.33 Using a Transwell system, the 2 engineered IgG3 antibodies were added to individual wells in parallel with the wild-type α-D (IgG1) at the apical (A) side. During a 26-hour time course, samples were collected from the basolateral (B) side, and the B:A ratios of the antibodies were quantified as shown in Figure 4. The antibodies were transferred across the BeWo monolayer of cells with B:A ratios of ∼0.1% (IgG3ΔHinge), 0.2% (α-D [IgG1]), and 0.3% (IgG3ΔHinge:R435H), resulting in a hierarchy as follows: IgG3ΔHinge:R435H>wild-type IgG1≥IgG3ΔHinge. The B:A ratios at the different time points revealed a statistically significant difference in transcytosis efficacy for IgG3ΔHinge and IgG3ΔHinge:R435H, whereas no significant difference was detected between the wild-type α-D (IgG1) and the engineered IgG3 ΔHinge variants.
BeWo cell monolayer transfer of wild-type IgG1 and engineered antibody. Antibody (α-D IgG1, IgG3ΔHinge, or IgG3ΔHinge:R435H) was added to the apical side of individual wells and medium fractions were collected from the apical and the basolateral reservoirs. The amount present in each fraction was measured by ELISA for quantitation of IgG and transfer was expressed as the basolateral:apical (B/A) ratio. Transfer of IgG3ΔHinge:R435H is significantly larger than transfer of IgG3ΔHinge.
BeWo cell monolayer transfer of wild-type IgG1 and engineered antibody. Antibody (α-D IgG1, IgG3ΔHinge, or IgG3ΔHinge:R435H) was added to the apical side of individual wells and medium fractions were collected from the apical and the basolateral reservoirs. The amount present in each fraction was measured by ELISA for quantitation of IgG and transfer was expressed as the basolateral:apical (B/A) ratio. Transfer of IgG3ΔHinge:R435H is significantly larger than transfer of IgG3ΔHinge.
Transport across ex vivo placenta
In contrast to monolayers of BeWo cells, the placenta is a complex organ consisting of several cell types and layers that maternal IgG must diffuse through or be transported across prior to delivery to the fetus. To better mimic placental transfer, we used a human ex vivo placental perfusion model as previously established.21 Equal amounts (10 µg/mL) of α-MSP-3 (wild-type IgG1 and IgG3), α-D (wild-type IgG1, IgG3ΔHinge, and IgG3ΔHinge:R435H) were added to the maternal reservoir in pairs of 1 engineered antibody together with 1 wild-type, the latter serving as standard. Samples were collected from the fetal side as indicated in supplemental Methods. All antibodies were transferred across the placenta except from IgG3ΔHinge that did not deviate from background at any point during the perfusion (P > .6). Background was defined as the average fetal value obtained from all perfusions between time zero and 60 minutes as stated in “Methods.” After 6 hours perfusion, the fetal concentrations of the 2 wild-type IgG1 antibodies, α-MSP-3 (IgG1) and α-D (IgG1), were 10.0 ± 9 µg/L and not statistically different (Figure 5A), demonstrating that transplacental transport was not affected by antibody specificity.
Placental ex vivo transport of wild-type IgG1 and engineered antibody. Antibodies were added to the maternal side in pairs of 1 wild-type and 1 engineered antibody. Medium fractions were collected from the fetal and maternal side at the indicated time points. Measurement of the concentration of antibody was done with an ELISA with the antigen for α-MSP-3 and in SOL-ELISA with D-positive RBCs for α-D, respectively. No difference was found in the placental transport kinetics of (A) α-MSP-3,IgG1 and α-D,IgG1, (B) α-D,IgG1 and α-MSP-3,IgG3, and (D) α-MSP-3,IgG1 and IgG3ΔHinge:R435H. In contrast, the fetal concentration of (C) IgG3ΔHinge was not significantly different from 0 µg/mL at any time point during the perfusion, demonstrating lack of transport.
Placental ex vivo transport of wild-type IgG1 and engineered antibody. Antibodies were added to the maternal side in pairs of 1 wild-type and 1 engineered antibody. Medium fractions were collected from the fetal and maternal side at the indicated time points. Measurement of the concentration of antibody was done with an ELISA with the antigen for α-MSP-3 and in SOL-ELISA with D-positive RBCs for α-D, respectively. No difference was found in the placental transport kinetics of (A) α-MSP-3,IgG1 and α-D,IgG1, (B) α-D,IgG1 and α-MSP-3,IgG3, and (D) α-MSP-3,IgG1 and IgG3ΔHinge:R435H. In contrast, the fetal concentration of (C) IgG3ΔHinge was not significantly different from 0 µg/mL at any time point during the perfusion, demonstrating lack of transport.
Next, perfusion of α-MSP-3 (IgG3) mixed with α-D (IgG1) showed transport of both antibodies with no statistically significant difference (Figure 5B). In contrast to the lack of transport of IgG3ΔHinge (Figure 5C), the IgG3ΔHingeR435H mutant was transported at a rate indistinguishable from the positive control antibody α-MSP-3 (IgG1) (P > .2) (Figure 5D). Thus, a hierarchy as follows was observed: IgG3ΔHinge:R435H=wild-type IgG1=wild-type IgG3>>>IgG3ΔHinge. The transport rate across the placenta was higher (0.1% per 6 hours) than that measured using BeWo cells (0.2% per 26 hours).
Endogenous maternal IgG antibodies residing in fetal and maternal tissues and vessels may compete with recombinant antibodies for transplacental transport.10 Therefore, we quantified the total amounts of IgG antibodies in the maternal and fetal circulation, where the mean maternal concentration of 16 individual representative placenta perfusions was 600 µg/mL after 6 hours of perfusion, whereas the fetal concentration reached an average value of 28 µg/mL (Figure 6). Thus, the maternal concentration is 60-fold higher than the concentration of the added recombinant IgG antibody (10 µg/mL). We observed no negative correlation between concentration of endogenous IgG in the maternal and fetal media and placenta transport of recombinant antibodies. On the contrary, a positive correlation between fetal endogenous IgG concentration and fetal recombinant IgG concentration was observed, suggesting that the individual placental variations of transport efficiency have a similar effect on recombinant and endogenous antibodies.
Quantification of the total IgG concentrations in placental perfusions. Total antibody concentration in maternal circulation and 10 times total antibody concentration in fetal circulation. Medium fractions were collected from the fetal and maternal side. Measurement of the concentration of antibody was done with an ELISA for IgG. Values shown are the averages of 16 experiments.
Quantification of the total IgG concentrations in placental perfusions. Total antibody concentration in maternal circulation and 10 times total antibody concentration in fetal circulation. Medium fractions were collected from the fetal and maternal side. Measurement of the concentration of antibody was done with an ELISA for IgG. Values shown are the averages of 16 experiments.
Discussion
In this study, we investigated 2 recombinant engineered human IgG3 variants that are transported differently across the human placenta. While the maternofetal transport rate of IgG3ΔHinge:R435H in the placenta model was shown to be equivalent to that of IgG1, IgG3ΔHinge was not transported. Strikingly, this difference is due only to a single point mutation within the CH3 domain of the IgG3 antibody, where an arginine is replaced by a histidine. The constructed human IgG3ΔHinge:R435H resembles the rare IgG3 allotype G3m(s,t), which has H435, and a serum half-life similar to that of IgG1, in contrast to R435-containing IgG3 allotypes.10 Furthermore, deletion of the hinge region and introduction of the C131S mutation were shown to completely eliminate binding of both IgG3ΔHinge and IgG3ΔHinge:R435H to all human classical FcγRs as well as C1q. This is in agreement with previous studies showing that these antibodies were inactive in both complement-dependent cytotoxicity and ADCC.18,19
However, IgG3ΔHinge as well as IgG3ΔHinge:R435H bound hFcRn in a strictly pH-dependent manner, similarly to that of wild-type IgG1 and IgG3, as demonstrated using ELISA; the lack of transport of IgG3ΔHinge in the placenta perfusion model is thus not caused by a lack of hFcRn binding. The gain of placental transfer achieved by introducing R435H, making IgG3ΔHinge:R435H more IgG1-like, may be explained by a subtle change in binding kinetics for hFcRn not reflected in the ELISA. This is supported by a recent study showing improved transcytosis and extended serum half-life of a recombinant IgG3 variant containing the R435H mutation. The finding was explained by IgG1 having a twofold higher binding affinity toward hFcRn at acidic pH compared with IgG3, and IgG3 having slightly higher binding affinity at neutral pH than IgG1, as revealed by kinetic determinations using surface plasmon resonance.10 Thus, although we measured in ELISA that IgG3ΔHinge binds hFcRn equally well as IgG3ΔHinge:R435H, it may be that minor but distinct differences in binding kinetics give rise to divergent transport activities of these 2 IgG3 antibodies across the placenta in the presence of endogenous IgG.
Stapleton et al used trophoblast-like cells or cells transfected with the gene for hFcRn seeded in a Transwell system and demonstrated that IgG1 and IgG3 antibodies were transcytosed equally well if they were added individually to the cells. However, when IgG1 and IgG3 were mixed, transfer of IgG3 was inhibited due to more favorable binding of IgG1 to hFcRn.10 This effect was not seen using an IgG3 mutant with R435H. Our data from the BeWo model were obtained with antibodies added individually to the cells, and we observed transcytosis of both IgG3ΔHinge and IgG3ΔHinge:R435H, with the latter being transported more efficiently.
We found a complete absence of transport of IgG3ΔHinge in the placenta perfusion model. In contrast, IgG3ΔHinge:R435H was transported. In both cases, a 60-fold excess of maternal IgG was present, which mainly consists of IgG1. Furthermore, wild-type IgG3 and IgG1 were transported equally well. Also, other studies have addressed the in vivo ratios between maternal and fetal concentrations of IgG1 and IgG3, and do not corroborate that in vivo IgG3 transplacental transport is inhibited, although this is a study on Japanese individuals probably involving G3m(st) allotypes.34 Therefore, to explain the inferior transport capacity of IgG3ΔHinge in the placenta model, it is warranted to implicate the hinge deletion in combination with the arginine in position 435.
The hinge-deleted IgG3 variants lost binding to all classical human FcγRs, while pH-dependent binding to FcRn was retained. The structural background for this is readily explained by inspection of crystal structures of the FcRn-Fc and FcγR-Fc complexes that show how these 2 classes of receptors bind to separate parts of the IgG molecule: the FcγRs to the lower hinge-CH2 region and FcRn to the junction of the CH3-CH2 domains.35,36 In addition, the engineered variants did not bind C1q, which partially share binding site with the FcγRs.
A dual receptor transport model has previously been suggested for IgG, with FcRn located in the syncytiotrophoblast being responsible for the initial transcytosis of IgG, and FcγRIIb residing in the endothelium of fetal vessels being responsible for the final transcytosis across fetal endothelium into fetal circulation.37 Such a model is not compatible with our observations; rather our data from IgG3ΔHinge:R435H suggest that FcRn is capable of completing the transfer from maternal to fetal circulation. However, conflicting data exist on the expression of classical FcγRs in placenta-derived cells.38 The b2 isoform of human FcγRII has been suggested to be involved in transcytosis of IgG across fetal endothelial cells,38 but direct evidence for involvement of FcγRIIb2 in placental transport is lacking, and a recent study in mice did not demonstrate a role for the mouse homolog of FcγRIIb in transport of IgG across the yolk sac.39,40 We conclusively show that IgG3ΔHinge:R435H, which completely lacks binding to classical FcγRs, is transcytosed equally as well as the wild-type IgG1; we therefore conclude that binding to hFcRn is sufficient for efficient and complete placental transfer.
Based on this conclusion, it was surprising to observe that IgG3ΔHinge and wild-type IgG3 had strikingly different transport rates in the placenta model. Both molecules have arginine at position 435; therefore, binding to FcRn is similar for the 2 molecules as demonstrated in the receptor-binding assay. The only difference between the 2 molecules is in the hinge region, which is clearly reflected in the differential binding to classical FcγRs. Our data from IgG3 suggest that a significant contribution to transfer is provided directly or indirectly by the hinge region. Future studies should address the transport routes for FcRn-mediated IgG delivery in placenta-derived cells or tissues, using advanced imaging technologies, similar to that previously reported for tracking of FcRn-mediated IgG transport across epithelial cells using electron tomography imaging techniques or multifocal plane microscopy.41,42
One other study has previously aimed to develop a recombinant antibody without effector functions that can cross the placenta; the CH2 sequences from IgG2 and IgG4 were grafted into an IgG1 background and eliminated cytotoxic activity.43 Subsequently, 3 amino acid residues responsible for allotypic immune responses were mutated (Lys214Thr, Asp356Glu, and Leu358Met), and evaluation in an ex vivo placenta model showed reduced transport compared with wild-type IgG.44 However, binding to FcRn was not addressed.
Thus, IgG3ΔHinge:R435H is the first antibody without effector functions, and with retained FcRn-binding properties, which has a documented transplacental transport similar to IgG1.
Vaccaro et al have described an Fc-engineered human IgG1 variant where 2 residues (H433 and N434) in proximity to H435 at the core of the FcRn-Fc interaction interface were targeted by mutagenesis (H433K/N434F), which resulted in considerably improved binding to FcRn at acidic pH without altered binding at neutral pH.45 This IgG1 variant was shown to be transported more efficiently from the maternal to fetal compartment than the wild-type counterpart. Both H433 and N434 are conserved among all human IgG subclasses, and thus introduction of the 2 mutations (433K and 434F) into IgG3ΔHinge:R435H may well improve transplacental transfer beyond that of wild-type IgG1.45
Clinically, IgG3ΔHinge:R435H could be useful for inhibition of a destructive process. More specifically, the anti-D specificity of the tested IgG would make it useful in treatment of HDFN where fetal anemia is caused by maternal anti-D antibodies that opsonize the RBCs. In this situation, the primary objective is to sustain the life of the fetus approximately to gestational week 34 at which time the infant can be delivered with an acceptable survival rate. Therefore, a complete or partial inhibition of hemolysis is highly beneficial (supplemental Figures 8-9). The present clinical practice is intrauterine transfusion of compatible donor blood through the umbilical cord vein.12 This is associated with a small but significant risk of fetal distress.46 Intravenous administration of IgG3ΔHinge:R435H to the mother would eliminate this risk. Furthermore, our concept would offer a treatment of the rare cases of hemolysis that occur before gestational week 20 when intrauterine transfusion is not feasible because of the dimensions of the fetal veins.
In conclusion, we have described a hinge region–deleted IgG3 antibody without effector functions that is capable of crossing the placenta at a rate similar to that of wild-type IgG1 antibodies. The present study also demonstrates the potential for designing antibodies that target other alloimmune diseases, such as FNAIT47 where effector functions are not desirable.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ‘‘advertisement’’ in accordance with 18 USC section 1734.
Acknowledgments
Jeanette Kolstrup Søgaard Nielsen assisted in placental perfusions, Marie Sønnegaard Poulsen assisted in the BeWo model transfer studies, and Betina Poulsen assisted in endogenous antibody measurements. We are also grateful to the staff at the maternity ward of Rigshospitalet and to the parents who donated placental material.
The initial work was supported by EU BioMed program contract number BMH4-CT96-1545, and by Toyota fonden (Denmark). J.T.A. was supported by the Norwegian Research Council (grant no. 179573/V40) and South-Eastern Norway Regional Health Authority (grant no. 39375).
Authorship
Contribution: L.M. designed the research, performed the research, performed the statistical analyses, analyzed and interpreted the data, and wrote the manuscript; L.K.N. and J.T.A. designed the research, performed the research, analyzed and interpreted the data, and wrote the manuscript; A.G. performed the research; I.S. designed the research, and analyzed and interpreted the data and participated in writing of the manuscript; T.E.M. analyzed and interpreted the data and participated in writing the manuscript; M.H. contributed vital components of the experimental system; L.E.K. designed the research and analyzed and interpreted the data; and M.H.D. designed the research, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: L.K.N. and M.H.D. hold a patent on the α-MSP-3 (IgG1 and IgG3), also termed IgG1+RAM1 and IgG3+RAM1, against the malaria antigen MSP-3 from Plasmodium falciparum, and used as a wild-type control in this article. The remaining authors declare no competing financial interests.
Correspondence: Morten Hanefeld Dziegiel, Blodbanken Section 2034, Rigshospitalet, Blegdamsvej 9, DK-2100 Copenhagen, Denmark; e-mail: dziegiel@rh.regionh.dk.
References
Author notes
L.K.N. and J.T.A. contributed equally to this study.