In this issue of Blood, Stowell et al describe a novel mouse model of hemolytic disease of the fetus and newborn (HDFN) that recapitulates many of the key features of human disease.1 Recently, this same group of researchers described a transgenic mouse that expresses the human KEL2 (Chellano) red cell surface protein from the Kell system on red cells,2 and subsequently demonstrated that Kell differences on transfused blood induce antibody responses and hemolytic transfusion reactions similar to those seen in patients.3 In this latest report, Stowell et al1 demonstrate that similar to some patients, Kell differences between mother and father can lead to maternal antibody generation and hemolytic disease in utero. In so doing, they provide experimental confirmation of a long sought after animal model of HDFN.
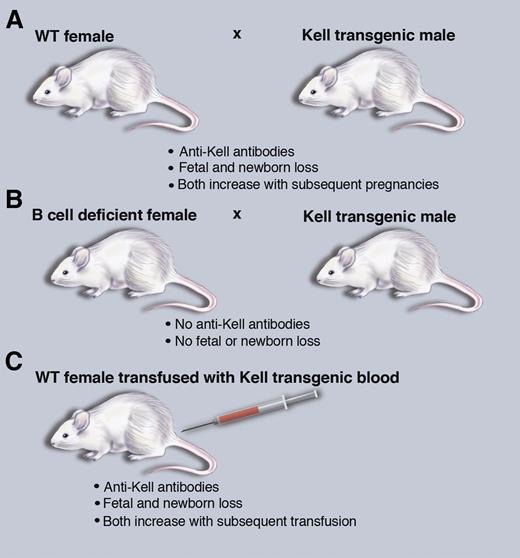
A mouse model of HDFN that recapitulates human disease. (A) Breeding wild-type (WT) female mice with Kel2 transgenic male mice leads to HDFN. (B) HDFN depends on B-cell production of antibodies. (C) Transfusion of Kel2 transgenic blood into wild-type females also leads to HDFN. Professional illustration by Marie Dauenheimer.
A mouse model of HDFN that recapitulates human disease. (A) Breeding wild-type (WT) female mice with Kel2 transgenic male mice leads to HDFN. (B) HDFN depends on B-cell production of antibodies. (C) Transfusion of Kel2 transgenic blood into wild-type females also leads to HDFN. Professional illustration by Marie Dauenheimer.
HDFN can be thought of as a clinically relevant natural experiment in human immunology, whereby allogenic paternal proteins expressed on red blood cells in the fetus are capable of elucidating a class switched, IgG alloantibody response in the mother.4 Exposure to red cell antigens typically occurs at the time of delivery, sparing the initial pregnancy. However, with subsequent pregnancies, transfer of maternal IgG to the developing fetus leads to antibody binding and destruction of developing fetal red blood cells. Alternatively, exposure of women of child-bearing age to paternal red cell antigens via transfusion can lead to antibody production capable of impacting even the first pregnancy. Classically, antibody titers and disease severity both increase with subsequent pregnancies; with lower titers associated with neonatal anemia and hyperbilirubinemia and higher titers associated with erhythroblastosis fetalis and interuterine death. Stowell et al1 convincingly demonstrate in their Kell-expressing transgenic mouse model that repeated exposure of Kell negative moms, via either multiple pregnancies or transfusion, leads to increasing anti-Kell antibody titers and worsening of HDFN of their offspring (see figure). By using mothers that are genetically incapable of antibody production, they definitively demonstrate that these antibodies drive the observed pathology (see figure).
Despite the initial description of HDFN in 1609 by a French midwife and the discovery of the antibody-mediated disease mechanism underlying it in the 1940s,4 our understanding of the basic immune mechanisms that govern induction of antibody responses in the relatively immunosuppressive environment of pregnancy remains unclear. The new Kell transgenic system described by Stowell et al1 provides an experimentally tractable model in which to address basic questions regarding the molecular mechanisms of initial immune sensitization. Perhaps more importantly, their work provides a clinically relevant model in which to investigate novel methods for prevention and treatment of HDFN going forward. Although polyclonal Rh(D) antisera (RhoGam) has proven tremendously successful in the prevention of the most common setting of HDFN driven by the Rh-D antigen, the mechanism of action of RhoGam remains a mystery.5 There are also many other RBC antigen systems capable of inducing severe HDFN for which there is no RhoGam equivalent, including the Kell, Kidd, and the non-D Rh antigens.6 In these non-D Rh mismatch settings, there is no proven prevention strategy and clinical management often requires interuterine transfusion strategies that themselves come with significant risk of fetal loss. Given its continued clinical importance, there have been several attempts throughout the years to generate animal models of HDFN. These attempts have focused on Rh(D) expression in one form or another, and they have universally proved unsuccessful, likely secondary to the combined effects of the genetic complexity of the Rh system7 and differences between mouse and human MHC class II peptide presentation. By focusing on the genetically less complicated, yet clinically relevant Kell system, Stowell et al1 have finally overcome this important hurdle. In so doing, they have provided a pre-clinical model in which development and testing of novel therapeutic approaches for the prevention and/or treatment of this devastating disease is now possible.
Conflict-of-interest disclosure: The authors declare no competing financial interests.