Key Points
In older patients with AML who are not suitable for intensive treatment, clofarabine doubles remission rates
Survival is not improved compared with low-dose Ara-C
Abstract
Better treatment is required for older patients with acute myeloid leukemia (AML) not considered fit for intensive chemotherapy. We report a randomized comparison of low-dose Ara-C (LDAC) vs the novel nucleoside, clofarabine, in untreated older patients with AML and high-risk myelodysplastic syndrome (MDS). A total of 406 patients with de novo (62%), secondary disease (24%), or high-risk MDS (>10% marrow blasts) (15%), median age 74 years, were randomized to LDAC 20 mg twice daily for 10 days every 6 weeks or clofarabine 20 mg/m2 on days 1 to 5, both for up to 4 courses. These patients had more adverse demographics than contemporaneous intensively treated patients. The overall remission rate was 28%, and 2-year survival was 13%. Clofarabine significantly improved complete remission (22% vs 12%; hazard ratio [HR] = 0.47 [0.28-0.79]; P = .005) and overall response (38% vs 19%; HR = 0.41 [0.26-0.62]; P < .0001), but there was no difference in overall survival, explained by poorer survival in the clofarabine patients who did not gain complete remission and also following relapse. Clofarabine was more myelosuppressive and required more supportive care. Although clofarabine doubled remission rates, overall survival was not improved overall or in any subgroup. The treatment of patients of the type treated here remains a major unmet need. This trial was registered at www.clinicaltrials.gov as #ISRCTN 11036523.
Introduction
An important proportion of older patients with acute myeloid leukemia (AML) do not receive conventional chemotherapy with a “3+7” or equivalent approach. Population studies indicate that this may be as many as 60% to 70% of patients.1,2 Epidemiological studies carried out in Sweden indicate that the survival was better in the localities where older patients tended to receive an intensive approach than in those locations where patients received a nonintensive or palliative approach.3 Because the reasons why patients were allocated to one or other treatment approach was not documented, this information does not provide complete evidence to support one or another approach. In a small randomized study that tested a policy of immediate vs delayed intensive therapy, there was no difference in survival.4 In our UK LRF AML14 trial, we attempted to address the issue of which patients should receive an intensive or a nonintensive strategy, hoping to recruit sufficient patients to define subsets in which one or another approach could be recommended. Out of a total recruitment of 1600 patients, only 8 were randomized.5 In a multivariate analysis, one of the most significant factors associated with the allocated treatment was the treating doctor, which suggested that physician preference plays an important role in this decision. In this trial, we established that low-dose Ara-C (LDAC) was superior to supportive care with no evidence of extra toxicity or supportive care required.6 The schedule of Ara-C 20 mg twice daily for 10 days is not satisfactory treatment, except for the 15% to 20% who enter remission where the median survival is 15 months, but it remains to be beaten by alternatives in a randomized comparison. Popular alternatives include demethylation agents, but they have not been proven to be superior to this schedule of LDAC, either because there was insufficient power to detect a difference in the 20% to 30% blast population7 or because a single daily dose was used.8 Because there is an urgent need to improve treatment in this patient population and there are a number of potential novel agents or combinations emerging, we developed a program in which we designated “Pick a Winner,” which was aimed at rapidly identifying new treatments for this patient population that were capable of making a clinically worthwhile improvement in outcome. This has been described in detail elsewhere.9 We report here the Pick-a-Winner comparison of clofarabine vs LDAC as part of the UK National Cancer Research Institute AML16 trial (ISRCTN 11036523).
Clofarabine (2-chloro-2′-fluoro-deoxy-9-β-d-arabinofuranosyladenine) was designed to incorporate the beneficial properties of fludarabine and cladribine, which are active as single agents in AML but at dose levels associated with prohibitive toxicity due to the cleavage product 2-fluoroadenine being converted to the toxic 2-fluoroadenosine.10,11 Clofarabine is the result of a program of development exploring a series of chemical modifications to minimize cleavage while retaining activity.12 It depends on membrane nucleoside transporters for cell entry and is sequentially phosphorylated in deoxycytidine-kinase–dependent steps to the triphosphate, the active form of which is retained within cells for longer than other purine nucleoside analogs. Following initial studies in relapsed disease that confirmed its activity,13 2 reports incorporating 3 unrandomized studies14,15 assessed the front-line activity in older patients using lower doses. These studies were consistent is delivering complete remission (CR) to more than 40% of patients, and of interest this responsiveness did not seem to be limited by age or cytogenetic risk group. It also had the potential of being orally available. Clofarabine was therefore identified as a candidate to be included in the Pick-a-Winner program to be randomized against LDAC.
Patients and methods
Patients with AML (de novo or secondary) and high-risk myelodysplastic syndrome (MDS) (>10% marrow blasts) could enter the study. During the study, all centers had within the protocol the option to receive an intensive chemotherapy approach and the reasons why patients were not treated intensively, and details of comorbidity (using the Sorror index16 components) were collected at entry. Patients could be randomized between LDAC and any of the concurrently available options for which they were eligible. To enter the LDAC vs clofarabine randomization, patients had to have renal function test results within the local upper limit of normal. Patients with acute promyelocytic leukemia or blast transformation of chronic myeloid leukemia were excluded. Diagnosis and response definitions were assessed by the local investigator. Cytogenetic and immunophenotypic characterization was carried out in regional reference laboratories that participate in national quality assurance schemes. Patients were required to give written consent and the trial was approved by the Wales Research Ethics Committee in compliance with the Declaration of Helsinki.
LDAC was given as a twice-daily 20-mg subcutaneous injection for 10 days with the aim of delivering 4 courses at approximately 6-week intervals, and clofarabine was given as 20 mg/m2 daily for 5 days by intravenous infusion for 4 courses approximately 4 to 6 weeks apart. Patients who were considered to be benefiting (CR or stable disease) were permitted to receive additional courses of treatment.
Adverse events and toxicity were recorded as defined by the National Cancer Institute Common Terminology Criteria version 3.
Statistical considerations
Definitions of end points
The protocol defined CR as a normocellular bone marrow aspirate containing <5% leukemic blasts and showing evidence of normal maturation of other marrow elements. Persistence of myelodysplastic features did not preclude the diagnosis of CR. Although not in the original protocol, in this report, to achieve CR, patients required neutrophil recovery to 1.0 × 109/L and platelets to 100 × 109/L, without evidence of extramedullary disease. Patients who achieved CR according to the protocol, but without count recovery, are denoted here as CRi. Assessment of remission status was undertaken after each treatment course until remission status was confirmed.
Following the international guidelines,17 overall survival (OS) is defined as the time from randomization to death. For remitters, survival from CR is defined as the time from CR/CRi (first report) until death. Relapse-free survival is the time from remission to either death or relapse, whichever occurs first. Surviving patients are censored at date last seen, and follow-up is complete to January 1, 2012. Median follow-up for survival is 25 months (range, 1-53 months). Survival percentages are quoted at 2 years.
Statistical methods
All analyses are by intention to treat. Categorical end points (eg, CR rates) were compared using Mantel-Haenszel tests, giving Peto odds ratios and confidence intervals. Continuous variables were analyzed by parametric (Student t test) or nonparametric (Wilcoxon rank sum) tests as appropriate. Time-to-event outcomes were analyzed using the log-rank test with Kaplan-Meier survival curves. Odds ratios (ORs) and hazard ratios (HRs) <1 indicate benefit for clofarabine over LDAC. Effect sizes are given with 95% confidence intervals throughout.
In addition to overall analyses, subgroup analyses were performed by the randomization stratification parameters and other important variables, with suitable tests for interaction. Because of the well-known dangers of subgroup analysis, these were interpreted cautiously.
Under the rules of the Pick-a-Winner design, the Data Monitoring and Ethics Committee (DMEC) would confidentially examine data once CR information was complete for 100 patients. In order to proceed, there needed to be a 2.5% improvement or greater in overall remission rate (CR + CRi). This occurred in September 2008, when the remission rates were respectively 34% (clofarabine) vs 15% (LDAC). The trial then continued until 200 patients were accrued, at which point there needed to be at least a 7.5% improvement in overall remission rate. At this assessment, in March 2010, the remission rates were 41% versus 21%. The DMEC therefore recommended completion of the trial with 200 patients in each arm, sufficient to give 80% power to detect a doubling of 2-year survival, from 11% (as seen in our AML14 trial) to 22% at P < .01.
Results
Between August 2006 and April 2011, 406 patients entered the randomization from 109 centers in the UK, Denmark, and Australia, with a median age of 74 years (range, 51-90 years) (Table 1). Figure 1 shows a CONSORT diagram of the disposition of the patients. Sixty-two percent had de novo disease, 24% secondary AML, and 15% high-risk MDS (>10% blasts); 2% had favorable cytogenetics, 72% intermediate, and 26% adverse. By Wheatley score,18 3% were good, 46% standard, and 51% poor risk; 13% had a WHO performance score ≥2. The characteristic of the randomized patients and contemporaneous patients given intensive chemotherapy in participating centers is shown in Table 1, which confirms that the patients in this study were significantly different from those entering the intensive treatment approach, even though cytogenetic data were not available to the investigator at entry.
Characteristics of patients randomized and comparison with contemporaneous intensive patients
| Characteristic . | LDAC (n = 206) . | Clofarabine (n = 200) . | Intensive (n = 1507) . | P value vs intensive . |
|---|---|---|---|---|
| Age (y) | ||||
| <60 | 3 | 3 | 24 | <.0001* |
| 60-64 | 9 | 5 | 412 | <.0001† |
| 65-69 | 30 | 26 | 595 | |
| 70-74 | 65 | 68 | 373 | |
| 75-79 | 60 | 73 | 96 | |
| ≥80 | 39 | 25 | 7 | |
| Median (range) | 74 (54-90) | 74 (51-87) | 67 (51-84) | |
| Sex | ||||
| Female | 86 | 75 | 586 | .8 |
| Male | 120 | 125 | 921 | |
| Diagnosis | ||||
| De novo | 126 | 124 | 1083 | .0003 |
| Secondary | 50 | 47 | 265 | |
| High-risk MDS | 30 | 29 | 159 | |
| WBC (× 109/L) | ||||
| <10 | 130 | 134 | 928 | .04* |
| 10-49.9 | 55 | 49 | 385 | .15† |
| 50-99.9 | 14 | 12 | 107 | |
| ≥100 | 7 | 5 | 87 | |
| Median | 4.6 (0.5-175.0) | 4.3 (0.5-260.6) | 5.1 (0.2-454.0) | |
| Performance status | ||||
| WHO PS 0 | 75 | 72 | 900 | <.0001* |
| WHO PS 1 | 106 | 100 | 510 | |
| WHO PS 2 | 16 | 22 | 60 | |
| WHO PS 3,4 | 9 | 6 | 37 | |
| Cytogenetics | ||||
| Favorable | 2 | 2 | 41 | .12* |
| Intermediate | 85 | 93 | 840 | |
| Adverse | 33 | 31 | 260 | |
| Unknown | 86 | 74 | 366 | |
| Wheatley group | ||||
| Good | 7 | 5 | 390 | <.0001* |
| Standard | 95 | 92 | 550 | |
| Poor | 104 | 103 | 567 | |
| FLT3-ITD | ||||
| Wild-type | 85 | 101 | 472 | .3 |
| Mutant | 20 | 9 | 93 | |
| Unknown | 101 | 90 | 942 | |
| NPM1 | ||||
| Wild-type | 85 | 95 | 390 | .6 |
| Mutant | 27 | 17 | 106 | |
| Unknown | 94 | 78 | 1021 | |
| Comorbidity | ||||
| Arrhythmia | 18/199 (9%) | 26/194 (13%) | 69/1456 (5%) | <.0001 |
| Cardiac | 49/202 (24%) | 53/193 (27%) | 174/1472 (12%) | <.0001 |
| Cerebrovascular | 8/202 (4%) | 11/195 (6%) | 58/1477 (4%) | .4 |
| Diabetes | 26/204 (13%) | 33/195 (17%) | 121/1481 (8%) | <.0001 |
| Mild hepatic | 13/203 (6%) | 10/195 (5%) | 68/1478 (5%) | .3 |
| Severe hepatic | 1/203 (<0.5%) | 3/195 (2%) | 21/1480 (1%) | .5 |
| Heart valve disease | 5/203 (2%) | 9/194 (5%) | 15/1472 (1%) | .0003 |
| Inflammatory bowel | 2/202 (1%) | 2/199 (1%) | 37/1475 (3%) | .07 |
| Infection | 27/204 (13%) | 30/194 (15%) | 193/1467 (13%) | .5 |
| Obesity | 14/204 (7%) | 20/195 (10%) | 95/1476 (6%) | .15 |
| Peptic ulcer | 3/203 (1%) | 5/195 (3%) | 21/1479 (1%) | .4 |
| Prior tumor | 22/204 (11%) | 20/195 (10%) | 100/1480 (7%) | .01 |
| Psychiatric | 7/198 (4%) | 6/195 (3%) | 32/1474 (2%) | .19 |
| Severe pulmonary | 3/201 (1%) | 3/193 (2%) | 15/1461 (1%) | .4 |
| Renal | 0/203 | 3/194 (2%) | 28/1480 (2%) | .11 |
| Rheumatologic | 19/203 (9%) | 18/195 (9%) | 83/1477 (6%) | .008 |
| Other | 92/193 (48%) | 89/183 (49%) | 425/1417 (30%) | <.0001 |
| Characteristic . | LDAC (n = 206) . | Clofarabine (n = 200) . | Intensive (n = 1507) . | P value vs intensive . |
|---|---|---|---|---|
| Age (y) | ||||
| <60 | 3 | 3 | 24 | <.0001* |
| 60-64 | 9 | 5 | 412 | <.0001† |
| 65-69 | 30 | 26 | 595 | |
| 70-74 | 65 | 68 | 373 | |
| 75-79 | 60 | 73 | 96 | |
| ≥80 | 39 | 25 | 7 | |
| Median (range) | 74 (54-90) | 74 (51-87) | 67 (51-84) | |
| Sex | ||||
| Female | 86 | 75 | 586 | .8 |
| Male | 120 | 125 | 921 | |
| Diagnosis | ||||
| De novo | 126 | 124 | 1083 | .0003 |
| Secondary | 50 | 47 | 265 | |
| High-risk MDS | 30 | 29 | 159 | |
| WBC (× 109/L) | ||||
| <10 | 130 | 134 | 928 | .04* |
| 10-49.9 | 55 | 49 | 385 | .15† |
| 50-99.9 | 14 | 12 | 107 | |
| ≥100 | 7 | 5 | 87 | |
| Median | 4.6 (0.5-175.0) | 4.3 (0.5-260.6) | 5.1 (0.2-454.0) | |
| Performance status | ||||
| WHO PS 0 | 75 | 72 | 900 | <.0001* |
| WHO PS 1 | 106 | 100 | 510 | |
| WHO PS 2 | 16 | 22 | 60 | |
| WHO PS 3,4 | 9 | 6 | 37 | |
| Cytogenetics | ||||
| Favorable | 2 | 2 | 41 | .12* |
| Intermediate | 85 | 93 | 840 | |
| Adverse | 33 | 31 | 260 | |
| Unknown | 86 | 74 | 366 | |
| Wheatley group | ||||
| Good | 7 | 5 | 390 | <.0001* |
| Standard | 95 | 92 | 550 | |
| Poor | 104 | 103 | 567 | |
| FLT3-ITD | ||||
| Wild-type | 85 | 101 | 472 | .3 |
| Mutant | 20 | 9 | 93 | |
| Unknown | 101 | 90 | 942 | |
| NPM1 | ||||
| Wild-type | 85 | 95 | 390 | .6 |
| Mutant | 27 | 17 | 106 | |
| Unknown | 94 | 78 | 1021 | |
| Comorbidity | ||||
| Arrhythmia | 18/199 (9%) | 26/194 (13%) | 69/1456 (5%) | <.0001 |
| Cardiac | 49/202 (24%) | 53/193 (27%) | 174/1472 (12%) | <.0001 |
| Cerebrovascular | 8/202 (4%) | 11/195 (6%) | 58/1477 (4%) | .4 |
| Diabetes | 26/204 (13%) | 33/195 (17%) | 121/1481 (8%) | <.0001 |
| Mild hepatic | 13/203 (6%) | 10/195 (5%) | 68/1478 (5%) | .3 |
| Severe hepatic | 1/203 (<0.5%) | 3/195 (2%) | 21/1480 (1%) | .5 |
| Heart valve disease | 5/203 (2%) | 9/194 (5%) | 15/1472 (1%) | .0003 |
| Inflammatory bowel | 2/202 (1%) | 2/199 (1%) | 37/1475 (3%) | .07 |
| Infection | 27/204 (13%) | 30/194 (15%) | 193/1467 (13%) | .5 |
| Obesity | 14/204 (7%) | 20/195 (10%) | 95/1476 (6%) | .15 |
| Peptic ulcer | 3/203 (1%) | 5/195 (3%) | 21/1479 (1%) | .4 |
| Prior tumor | 22/204 (11%) | 20/195 (10%) | 100/1480 (7%) | .01 |
| Psychiatric | 7/198 (4%) | 6/195 (3%) | 32/1474 (2%) | .19 |
| Severe pulmonary | 3/201 (1%) | 3/193 (2%) | 15/1461 (1%) | .4 |
| Renal | 0/203 | 3/194 (2%) | 28/1480 (2%) | .11 |
| Rheumatologic | 19/203 (9%) | 18/195 (9%) | 83/1477 (6%) | .008 |
| Other | 92/193 (48%) | 89/183 (49%) | 425/1417 (30%) | <.0001 |
PS, performance status.
Mantel Haenszel test for trend.
Wilcoxon rank sum test; all other tests by χ2 analysis.
The reasons for choosing a nonintensive treatment approach and the comorbidities recorded at entry are listed in Table 2. Age and a general assessment of “fitness” accounted for two-thirds of the reasons given. The comorbidities were significantly different in the randomized patients compared with those treated intensively but were balanced between the study arms. The main differences were cardiac, diabetes, rheumatologic, prior tumors, and inflammatory bowel disease (Table 1).
Reasons for choosing nonintensive therapy
| Reason . | LDAC . | Clofarabine . |
|---|---|---|
| Age | 134/205 (65%) | 136/196 (69%) |
| Fitness | 130/205 (63%) | 117/196 (60%) |
| Other | 29/204 (14%) | 18/196 (9%) |
| Patient choice (QoL) | 16 | 9 |
| Clinician decision | 1 | 1 |
| NI the only option at this center | 2 | 3 |
| Prior cancer | 2 | 0 |
| Heart disease | 3 | 3 |
| Hip replacement | 1 | 0 |
| COPD | 1 | 0 |
| Secondary disease | 1 | 0 |
| Psychiatric | 0 | 1 |
| Other | 3 | 1 |
| Reason . | LDAC . | Clofarabine . |
|---|---|---|
| Age | 134/205 (65%) | 136/196 (69%) |
| Fitness | 130/205 (63%) | 117/196 (60%) |
| Other | 29/204 (14%) | 18/196 (9%) |
| Patient choice (QoL) | 16 | 9 |
| Clinician decision | 1 | 1 |
| NI the only option at this center | 2 | 3 |
| Prior cancer | 2 | 0 |
| Heart disease | 3 | 3 |
| Hip replacement | 1 | 0 |
| COPD | 1 | 0 |
| Secondary disease | 1 | 0 |
| Psychiatric | 0 | 1 |
| Other | 3 | 1 |
COPD, chronic obstructive pulmonary disease; NI, nonintensive; QoL, quality of life.
Overall, 95% of patients received at least 1 course of treatment (LDAC 96%, clofarabine 94%), with 94% receiving their allocated treatment in course 1 (LDAC 95%, clofarabine 93%). The median number of courses for each group was 2 for both LDAC (mean, 3.0; range, 0-8) and clofarabine (mean, 2.1; range, 0-8); patients allocated to LDAC received more courses of treatment on average than clofarabine (P < .0001). Treatment continuation beyond 4 courses was given to 23 of 106 remitters (22%) and 23 of 114 patients (20%) with stable disease. Of patients allocated to clofarabine, 2, 1, 1, and 2 received 5, 6, 7, or 8 courses, while 7, 13, 4, and 16 LDAC patients received 5, 6, 7, and 8 courses, respectively. Only 1 patient allocated to clofarabine received more than 4 courses of clofarabine, the others receiving LDAC beyond course 4.
Remission induction
The overall response rate was significantly improved in the clofarabine arm (38% vs 19%; OR = 0.41 [0.26-0.62]; P < .0001), which included a superior rate of CR (22% vs 12%; OR = 0.47 [0.28-0.79]; P = .005) and CRi (16% vs 8%). The benefit was due to less disease resistance (44% vs 67%; OR = 0.39 [0.27-0.58]; P < .0001; Table 3). Of the remissions achieved in the clofarabine arm with data, 45% (17% of all patients with remission data) were recorded after 1 course and 55% (21% of patients) after 2 or more courses, whereas in the LDAC arm 23% of remissions (4% of patients) were recorded after course 1 and 73% of remissions (15% of patients) required 2 or more courses (P = .0005 for number of courses among remitters). The median time to CR was 113 days (LDAC) and 68 days (clofarabine), respectively.
Outcomes for patients by randomized allocation
| . | LDAC . | Clo . | HR/OR (95% CI) . | P value . |
|---|---|---|---|---|
| CR | 12% | 22% | 0.47 (0.28-0.79) | .005 |
| CRi | 8% | 16% | ||
| ORR (CR + CRi) | 19% | 38% | 0.41 (0.26-0.62) | <.0001 |
| Resistant disease | 67% | 44% | 0.39 (0.27-0.58) | <.0001 |
| Induction death | 13% | 18% | 1.42 (0.83-2.44) | .2 |
| 30-d mortality | 13% | 18% | ||
| 60-d mortality | 26% | 32% | ||
| 2-y survival | 12% | 13% | 0.96 (0.78-1.19) | .7 |
| 2-y RFS | 8% | 20% | 0.76 (0.49-1.19) | .2 |
| 2-y survival from CR | 44% | 26% | 1.19 (0.74-1.91) | .5 |
| 2-y survival from relapse | 8% | 0% | 1.91 (1.10-3.31) | .02 |
| 2-y survival for non-CR | 4% | 3% | 1.37 (1.06-1.76) | .02 |
| . | LDAC . | Clo . | HR/OR (95% CI) . | P value . |
|---|---|---|---|---|
| CR | 12% | 22% | 0.47 (0.28-0.79) | .005 |
| CRi | 8% | 16% | ||
| ORR (CR + CRi) | 19% | 38% | 0.41 (0.26-0.62) | <.0001 |
| Resistant disease | 67% | 44% | 0.39 (0.27-0.58) | <.0001 |
| Induction death | 13% | 18% | 1.42 (0.83-2.44) | .2 |
| 30-d mortality | 13% | 18% | ||
| 60-d mortality | 26% | 32% | ||
| 2-y survival | 12% | 13% | 0.96 (0.78-1.19) | .7 |
| 2-y RFS | 8% | 20% | 0.76 (0.49-1.19) | .2 |
| 2-y survival from CR | 44% | 26% | 1.19 (0.74-1.91) | .5 |
| 2-y survival from relapse | 8% | 0% | 1.91 (1.10-3.31) | .02 |
| 2-y survival for non-CR | 4% | 3% | 1.37 (1.06-1.76) | .02 |
CI, confidence interval; Clo, clofarabine; ORR, overall remission rate; RFS, relapse-free survival.
Toxicity and resource use
Grade 3 or 4 gastrointestinal and hepatic toxicity was significantly greater in patients given clofarabine (Table 4), but this was generally manageable. The supportive care requirements, days in hospital, and days on antibiotics were significantly greater in the clofarabine arm, reflecting the increased level of myelosuppression (Table 4).
Toxicity and resource usage by randomization arm
| Toxicity . | LDAC . | Clofarabine . | P value . |
|---|---|---|---|
| % grade 3-4 (mean grade) . | % grade 3-4 (mean grade) . | ||
| Course 1 | |||
| Nausea | 4% (0.5) | 9% (0.9) | <.0001 |
| Oral | 5% (0.5) | 7% (0.8) | 1.0 |
| Diarrhea | 0% (0.4) | 4% (0.4) | <.0001 |
| Cardiac | 6% (0.3) | 8% (0.5) | .005 |
| Liver AST | 3% (0.3) | 7% (0.6) | .16 |
| Liver ALT | 3% (0.4) | 9% (1.0) | <.0001 |
| Bilirubin | 3% (0.5) | 7% (1.1) | <.0001 |
| Mean blood units | 5.9 | 8.9 | <.0001 |
| Mean platelet units | 3.6 | 7.2 | <.0001 |
| Mean days on antibiotics | 7.1 | 11.6 | <.0001 |
| Mean nights in hospital | 13.4 | 20.3 | <.0001 |
| Course 2 | |||
| Nausea | 0% (0.4) | 2% (0.7) | .0004 |
| Oral | 2% (0.2) | 6% (0.5) | .02 |
| Diarrhea | 1% (0.4) | 1% (0.2) | .02 |
| Cardiac | 1% (0.1) | 2% (0.2) | .4 |
| Liver AST | 0% (0.2) | 12% (0.9) | .0002 |
| Liver ALT | 1% (0.3) | 9% (0.9) | <.0001 |
| Bilirubin | 1% (0.3) | 1% (0.6) | .0001 |
| Mean blood units | 5.4 | 6.0 | .12 |
| Mean platelet units | 2.7 | 4.1 | .004 |
| Mean days on antibiotics | 3.7 | 6.6 | .002 |
| Mean nights in hospital | 9.2 | 12.3 | .003 |
| Toxicity . | LDAC . | Clofarabine . | P value . |
|---|---|---|---|
| % grade 3-4 (mean grade) . | % grade 3-4 (mean grade) . | ||
| Course 1 | |||
| Nausea | 4% (0.5) | 9% (0.9) | <.0001 |
| Oral | 5% (0.5) | 7% (0.8) | 1.0 |
| Diarrhea | 0% (0.4) | 4% (0.4) | <.0001 |
| Cardiac | 6% (0.3) | 8% (0.5) | .005 |
| Liver AST | 3% (0.3) | 7% (0.6) | .16 |
| Liver ALT | 3% (0.4) | 9% (1.0) | <.0001 |
| Bilirubin | 3% (0.5) | 7% (1.1) | <.0001 |
| Mean blood units | 5.9 | 8.9 | <.0001 |
| Mean platelet units | 3.6 | 7.2 | <.0001 |
| Mean days on antibiotics | 7.1 | 11.6 | <.0001 |
| Mean nights in hospital | 13.4 | 20.3 | <.0001 |
| Course 2 | |||
| Nausea | 0% (0.4) | 2% (0.7) | .0004 |
| Oral | 2% (0.2) | 6% (0.5) | .02 |
| Diarrhea | 1% (0.4) | 1% (0.2) | .02 |
| Cardiac | 1% (0.1) | 2% (0.2) | .4 |
| Liver AST | 0% (0.2) | 12% (0.9) | .0002 |
| Liver ALT | 1% (0.3) | 9% (0.9) | <.0001 |
| Bilirubin | 1% (0.3) | 1% (0.6) | .0001 |
| Mean blood units | 5.4 | 6.0 | .12 |
| Mean platelet units | 2.7 | 4.1 | .004 |
| Mean days on antibiotics | 3.7 | 6.6 | .002 |
| Mean nights in hospital | 9.2 | 12.3 | .003 |
ALT, alanine transaminase; AST, aspartate transaminase.
Postinduction outcomes
Remission failures.
A total of 166 of the 206 patients (81%) in the LDAC arm and 123 of the 198 patients (62%) in the clofarabine arm with remission status failed to enter CR. Among these patients, the survival was worse in the clofarabine arm (median survival was 107 days for LDAC vs 60 days for clofarabine; HR = 1.37 [1.06-1.76]; P = .02; Figure 2A).
Outcomes by randomization arm. (A) Survival for patients not achieving remission. (B) Relapse-free survival. (C) Survival from relapse. (D) OS.
Outcomes by randomization arm. (A) Survival for patients not achieving remission. (B) Relapse-free survival. (C) Survival from relapse. (D) OS.
Duration of remission.
The median duration of remission/relapse-free survival in the 40 LDAC patients was not significantly different from the 75 clofarabine remitters (8% vs 20% at 2 years; HR = 0.76 [0.49-1.19]; P = .5; Figure 2B).
Survival from relapse.
The survival of the of the 31 LDAC patients who relapsed (median survival from relapse, 40 weeks) was significantly better than for the 41 clofarabine patients who relapsed (median survival, 20 weeks; HR = 1.91 [1.10-3.31]; P = .02; Figure 2C). Of the 41 patients on clofarabine who relapsed, 7 of 39 are known to have received further treatment, which was daunorubicin/Ara-C (n = 1) or cytarabine alone (n = 6). A higher proportion of LDAC patients (16 of 30; P = .002) who relapsed received further therapy, which was daunorubicin/Ara-C (n = 1), fludarabine/Ara-C/G-CSF (n = 1), Ara-C (n = 11), hydroxyurea (n = 1), etoposide (n = 1), or azacitidine (n = 1).
Overall survival.
Because the survival of LDAC patients who failed to enter CR or who relapsed from CR was superior to that of clofarabine patients and the duration of remission was not significantly inferior to clofarabine, the net result was that there was no OS difference between the arms (12% vs 13% at 2 years; HR = 0.96 [0.78-1.19]; P = .7; Figure 2D).
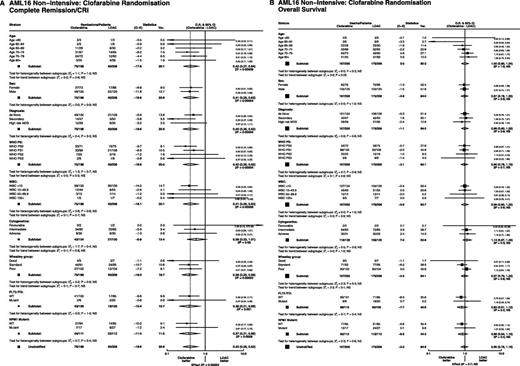
Exploratory subgroup analysis of demographics, cytogenetics, and molecular characteristics indicated that the beneficial effect on remission achievement could be seen in all subgroups (Figure 3A), but there was no subgroup in which a survival benefit could be found (Figure 3B).
Stratified analyses of remission and OS. (A) Overall remission rate. (B) OS.
Discussion
The older patient with AML presents a therapeutic dilemma. Although many studies have delivered conventional induction chemotherapy, which has successfully delivered a remission, most patients will relapse within a few months. This has led to a trend of loss of confidence in this approach, even in some cases for patients who are judged as likely to withstand intensive therapy. There are many patients who either do not wish to accept intensive treatment or who are advised that it represents an undue risk with little durable benefit. There are no universally agreed objective criteria to help with this decision. It was hoped that by randomizing this question in our AML14 trial, we would be able to identify such criteria. While age alone serves as a useful surrogate for predicting outcome, it is not, of itself, necessarily the correct parameter. However, there are many patients who are not treated with standard chemotherapy and in the past have been managed by best supportive care. A randomized comparison established that LDAC was superior to best supportive care without adding toxicity, thus suggesting LDAC as a standard of care, albeit far from ideal. Some patients benefit from therapy with demethylation agents, but they have not yet been shown in randomized studies to be superior to LDAC as delivered in the AML14 trial. New treatments are urgently required.
Clofarabine was clinically developed by the MD Anderson investigators and proved to be effective as monotherapy for the treatment of relapsed AML and ALL. Given its favorable toxicity profile, potential for oral administration, and similarity to fludarabine, it became a potential candidate novel therapy for the older patient. Three unrandomized phase 2 trials confirmed this hope and produced very similar results in that CR was seen in about 40% of patients with similar efficacy in patients under or over 70 years or with an adverse karyotype. These trials did not establish the duration of response. In one of these pilot trials, because of concerns about renal toxicity a dose level of 20 mg/m2 was tested in a small number of patients against the conventional 30-mg/m2 dose and produced a similar efficacy but with a more favorable toxicity profile. For this reason, the 20-mg/m2 dose was taken forward in this study. There therefore remains the possibility that the 30-mg/m2 dose could have been beneficial.
The study here reported is the only randomized trial comparing clofarabine monotherapy. According to the rules of Pick a Winner, the DMEC, in a confidential analysis, required at least a 2.5% improvement in remission rate in the first 100 randomized patients. This was achieved, and therefore the trial continued to recruit with OS as the primary end point. In spite of doubling the remission rate, the OS was not improved. There were a number of reasons for this. Recipients of clofarabine who failed to achieve CR survived less well than LDAC patients who did not enter CR. Survival in patients who achieved CR but relapsed survived less well in the clofarabine arm. It might be expected that a reason for not showing a survival benefit, having doubled the remission rate, was that clofarabine was able to achieve remissions in more resistant patients, which may result in a shorter duration of remission. This was not the case, because the relapse-free survival was similar in both arms. The increased remission rate was obtained at the “cost” of more toxicity and supportive care. The phase 2 trials suggested that the efficacy of clofarabine was seen irrespective of adverse cytogenetics or age, so it might have been expected that these subgroups at least would show benefit against LDAC. This was not the case.
Clofarabine is clearly an effective drug. Our assumption in judging a new agent is that if it delivers a better remission rate, then it should improve survival. But the evidence seen here is similar to that seen with the addition of gemtuzumab ozogamicin to LDAC, which also doubled the remission rate but did not improve survival.19 The experience with demethylation agents raises the possibility that, in older patients, it may be possible to improve survival without improving the rate of remission. It could, with some justification, be assumed that being in remission is preferable even though survival is not extended. In this randomization, quality-of-life information to support that assumption was not collected but is now incorporated in the Pick-a-Winner design. So the challenge is to maintain remission. While the 20-mg/m2 dose was well tolerated in this trial, it was only possible to deliver an average of 3 courses, even to those who entered remission, although survival was better for patients who received more courses (P = .0005 for trend over number of courses of treatment delivered). This raises the possibility that if more courses could be delivered, perhaps by lowering the dose, then it may be possible to prolong remission. Perhaps a priority should be to deliver effective treatment at a dose and periodicity that increase compliance.
Presented as an oral presentation at the 54th annual meeting of the American Society of Hematology, Atlanta, GA, December 10, 2012.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the physicians and patients who took part in the trial, Cancer Research UK for providing research funding, Genzyme/Sanofi for providing clofarabine and supporting the trial, the Cardiff Experimental Cancer Medicine Centre for mutation analysis and sample storage, and the Birmingham Clinical Trials Unit and the Haematology Clinical Trials Unit, Cardiff for trial support.
Authorship
Contribution: A.K.B. and R.K.H. designed the trial; A.K.B., N.H.R., A.E.H., D.M., S.K., J.Y., M.F.M., S.A., and D.B. provided patients to the trial; K.W. supervised data collection; R.K.H. performed the statistical analysis; and A.K.B. and R.K.H. drafted the paper, which was approved by all authors.
Conflict-of-interest disclosure: A.K.B. received research funding from Genzyme/Sanofi and acted on advisory boards for Genzyme/Sanofi; R.K.H. provided statistical advice for Genzyme/Sanofi. N.H.R. has acted a speaker at investigator meetings for Genzyme/Sanofi. The remaining authors declare no competing financial interests.
A list of physicians who entered patients into the trial appears in Appendix.
Correspondence: Alan Burnett, Department of Haematology, Cardiff University School of Medicine, Heath Park, Cardiff, United Kingdom CF14 4XN; e-mail burnettak@cardiff.ac.uk.
Appendix: physicians who entered patients into the trial
Aalborg Hospital: Mette Skov Holm and Maria Kallenbach; Aberdeen Royal Infirmary: D.J. Culligan; Addenbrooke’s Hospital: C. Crawley and J. Craig; Arrowe Park Hospital: D.W. Galvani, Nauman Butt, and Ranjit Dasgupta; Barnet General Hospital: A. Virchis, Marilyn Treacy, and Sylvia Berney; Belfast City Hospital: F. Jones, Mary Frances McMullin, and R.J.G. Cuthbert; Birmingham Heartlands Hospital: D.W. Milligan, G.E.D. Pratt, Richard Lovell, and Shankara Paneesha; Blackpool Victoria Hospital: P.A. Cahalin; Borders General Hospital: Ashok Okhandiar and J. Tucker; Bradford Royal Infirmary: A.T. Williams and Samuel Ackroyd; Bristol Haematology and Oncology Centre: S. Robinson; Canberra Hospital: James D’Rozario and Philip Crispin; Cheltenham General Hospital: R. Lush; Chesterfield and North Derbyshire Royal Hospital: M. Wodzinski and R. Stewart; Christie Hospital: M. Dennis; Churchill Hospital: P. Vyas; City Hospitals Sunderland: Lucy Pemberton, M.J. Galloway, Simon Lyons, Victoria Hervey, and Yogesh Upadhye; Colchester General Hospital: Gavin Campbell and Tendai Maboreke; Countess of Chester Hospital: E. Lee and Salaheddin Tueger; Crosshouse Hospital: Julie Gillies and M. Mccoll; Derbyshire Royal Infirmary: A. Mckernan and Cherry Chang; Dorset County Hospital: A.H. Moosa; Eastbourne District General Hospital: R.J. Grace; Falkirk and District Royal Infirmary: R.F. Neilson; Gartnavel General Hospital: Mark Drummond, Pam McKay, and Richard Soutar; Gloucestershire Royal Hospital: J. Ropner, Rebecca Frewin, and S. Chown; Hereford County Hospital: L.G. Robinson; Hillingdon Hospital: Ketan Patel and R. Kaczmarski; Hull Royal Infirmary: S. Ali and James Paget Hospital: Cesar Gomez and Shalal Sadullah; Kent and Canterbury Hospital: C.F.E. Pocock, F. Zwaan, K. Saied, and V. Ratnayake; Kent and Sussex Hospital: D. Gillett and Richard F. Gale; Kettering General Hospital: I. Wilson-Morkeh; King George Hospital: I. Grant; Leeds General Infirmary: G.M. Smith and Rod Johnson; Leicester Royal Infirmary: A.E. Hunter; Lincoln County Hospital: K. Saravanamuttu; Maidstone District General Hospital: D. Gillett, Richard F. Gale, and Saad Rassam; Medway Maritime Hospital: Maadh Aldouri and V.E. Andrews; Monklands Hospital: J.A. Murphy; Musgrove Park Hospital: S. Bolam; Norfolk and Norwich University Hospital: Matthew Lawes; Northampton General Hospital: A.L. Bowen and Jane Parker; Northwick Park Hospital: C.D.L. Reid and N. Panoskaltsis; Nottingham University Hospitals NHS Trust: E. Das-Gupta, J.L. Byrne, and N.H. Russell; Peterborough District Hospital: C. Hoggarth; Peterborough District Hospital: Sateesh Kumar Nagumantry; Pilgrim Hospital: V. Tringham; Pinderfields General Hospital: D. Wright, John Ashcroft, M.R. Chapple, and Paul Moreton; Poole General Hospital: A.J. Bell; Princess Alexandra Hospital: Tony Mills; Princess Royal University Hospital: A. Lakhani and C. De Lord; Queen Alexandra Hospital: C.M. James, H. Dignum, M. Ganczakowski, and R. Corser; Queen Elizabeth Hospital (Kings Lynn): N. Curtin; Queen Elizabeth Hospital Birmingham: J.A. Murray; Queen’s Hospital, Romford: Claire Hemmaway, I. Grant and Jane Stevens; Raigmore Hospital: C. Lush and William Murray; Rigshospitalet University Hospital: Jesper Jurlander, Lars Kjeldsen, Ole Weis Bjerrum, and Ove Juul Nielsen; Royal Berkshire Hospital: H. Grech; Royal Blackburn Hospital: Margaret Rokicka and Silvia Chernigoy; Royal Bournemouth General Hospital: Joseph Chacko and R. Hall; Royal Cornwall Hospital (Treliske): A.R. Kruger, Bryson Pottinger, E. Parkins, and M.D. Creagh; Royal Darwin Hospital: Ferenc Szabo; Royal Devon and Exeter Hospital (Wonford): M.V. Joyner and R. Lee; Royal Free Hospital: P. Kottaridis; Royal Gwent Hospital: Chris Jenkins; Royal Hobart Hospital: Rosemary Harrup and Ray Lowenthal; Royal Marsden Hospital (Surrey): M. Ethell; Royal Sussex County Hospital: Timothy Corbett; Royal United Hospital Bath: Chris Knechtli; Russells Hall Hospital: D. Bareford; Salford Royal Hospital: J.B. Houghton and Simon Jowitt; Salisbury District Hospital: J.O. Cullis and Tamara Everington; Sandwell General Hospital: F. Wandroo, John Gillson, and Y. Hasan; Scunthorpe General Hospital: S. Jalihal; Singleton Hospital: A. Benton, H. Sati, and S. Al-Ismail; Southampton General Hospital: D. Richardson and K. Orchard; Southern General Hospital: I. MacDonald; Southport and Formby District General Hospital: David O’Brien; St Bartholomew’s Hospital: Heather Oakervee and J. Cavenagh; St Helier Hospital: J. Mercieca; St James’s University Hospital: B.A. Mcverry and D.T. Bowen; Staffordshire General Hospital: P. Revell; Stirling Royal Infirmary: R.F. Neilson; Stoke Mandeville Hospital: A.M. O’Hea and A. Watson; Sykehuset Buskerud Trust: Jakob Dalgaard; The Alexandra Hospital: Elizabeth Maughan; The Alfred Hospital: Andrew Wei; The Great Western Hospital: A.G. Gray, Alex Sternberg, and N.E. Blesing; The James Cook University Hospital: Angela Wood, D. Plews, and R. Dang; The Royal Bolton Hospital: J. Jip and Mark Grey; The Royal Liverpool University Hospital: R.E. Clark; The Royal Oldham Hospital: Allameddine Allameddine and S. Elhanash; The Royal Victoria Infirmary: Gail Jones; University College Hospital: A. Khwaja; University Hospital Aintree: W. Sadik; University Hospital Coventry (Walsgrave): M. Narayanan; University Hospital of North Staffordshire: D. Chandra; University Hospital of North Tees: P. Mounter and Z. Maung; University Hospital of Wales: C. Poynton, C. Rowntree, Jonathan Kell, and S. Knapper; A.K. Burnett; Victoria Hospital: Kerri Davidson, Lorna McLintock, and Peter Williamson; West Middlesex University Hospital: M. Sekhar; Western General Hospital: P.H. Roddie and P.R.E. Johnson; Whiston Hospital: Toby Nicholson; Worcestershire Royal Hospital: N. Pemberton and S. Shafeek; Worthing Hospital: A.M. O’Driscoll; Wycombe General Hospital: R. Aitchison; York Hospital: L. Munro; Ysbyty Glan Clwyd: C. Hoyle and Earnest Heartin; Ysbyty Gwynedd: David R. Edwards and James Seale.