Key Points
We found no association between iron overload and survival or complications in allogeneic transplant recipients at 1 year posttransplant.
Future studies should use liver iron content to define iron overload instead of ferritin in this population.
Abstract
Using liver magnetic resonance imaging (R2-MRI) to quantify liver iron content (LIC), we conducted a prospective cohort study to determine the association between iron overload and adult allogeneic hematopoietic cell transplantation (HCT) outcomes. Patients received pretransplant ferritin measurements; patients with ferritin >500 ng/mL underwent R2-MRI. Patients were defined as no iron overload (N = 28) and iron overload (LIC >1.8 mg/g; N = 60). Median LIC in the iron-overload group was 4.3 mg/g (range, 1.9-25.4). There was no difference in the 1-year probability of overall survival, nonrelapse mortality, relapse, acute or chronic graft-versus-host disease, organ failure, infections, or hepatic veno-occlusive disease between groups. We also found no difference in the cumulative incidence of a composite end point of nonrelapse mortality, any infection, organ failure, or hepatic veno-occlusive disease (1-year cumulative incidence, 71% vs 80%; P = .44). In multivariate analyses, iron-overload status did not impact risks of overall mortality (relative risk = 2.3; 95% confidence interval, 0.9-5.9; P = .08). In conclusion, we found no association between pretransplant iron overload and allogeneic HCT outcomes. Future studies in this population should use LIC to define iron overload instead of ferritin.
Introduction
Several studies have reported associations between elevated ferritin levels, as a marker for iron overload, and adverse outcomes following allogeneic hematopoietic cell transplantation (HCT).1-7 However, it is well established that elevated serum ferritin is not specific for iron overload and ferritin levels do not provide an accurate estimation of body iron stores.8,9 Similarly, serum ferritin has been shown to be only modestly correlated with iron overload in allogeneic HCT recipients.10,11 Hence, it is unclear whether the association of elevated ferritin with adverse survival is primarily due to iron overload or to the presence of other infectious or inflammatory comorbidities for which ferritin might act as a surrogate indicator. Liver magnetic resonance imaging (R2-MRI) can estimate liver iron content (LIC) with high specificity and is a more accurate measure of iron overload compared with ferritin.12,13 To obtain a better understanding of the association between iron overload and outcomes following allogeneic HCT, we designed a prospective cohort study where we defined iron overload based on R2-MRI–measured LIC.
Materials and methods
Patients
Patients 18 to 75 years of age undergoing allogeneic HCT using myeloablative or reduced-intensity conditioning (RIC) regimens for any diagnosis were invited to participate in our single-institution prospective cohort study. Exclusion criteria included any contraindication to performing MRI and pregnancy. The study enrollment period ran from 2009 to 2011. All participants provided written informed consent to participate in the study in accordance with the Declaration of Helsinki, and the study was conducted under guidance of the University of Minnesota institutional review board.
Study design
All patients who consented to participate in the study underwent baseline serum ferritin measurements within 30 days prior to inpatient hospitalization for HCT conditioning. Patients with serum ferritin >500 ng/mL underwent pretransplant LIC determination using R2-MRI (normal range, 0.17-1.8 mg/g dry weight). Patients with LIC >1.8 mg/g were classified as “iron overload.” Patients with ferritin ≤500 ng/mL or ferritin >500 ng/mL and LIC ≤1.8 mg/g were classified as “no iron overload.” The MRI measurement of LIC was based on the imaging of proton transverse relaxation rates (R2) within the liver using a 1.5 T MRI machine (Siemens Magnetom Avanto, Malvern, PA). This noninvasive R2-MRI technique (FerriScan; www.resonancehealth.com) is highly sensitive and specific for estimating LIC and is approved by the Food and Drug Administration for routine clinical use.10,12-15 We used a conservative ferritin threshold of >500 ng/mL for conducting MRI assessment for LIC because the likelihood of iron overload below this ferritin cutoff is very low.10,11,14-16
Patients were followed up for 1 year after transplantation. Serial serum ferritin measurements were performed at 3, 6, 9, and 12 months after HCT. At 1 year posttransplantation, R2-MRI was repeated in patients with pretransplant iron overload and in patients with no pretransplant iron overload but 1-year ferritin level >500 ng/mL. Patients with high LIC did not receive any treatment for iron overload during the course of the study. Study-related data and end-point assessments were prospectively captured as part of our institution’s blood and marrow transplant database. A subset of patients also consented to participation in a separate immune reconstitution protocol. For these patients, peripheral blood was collected at 3, 6, and 12 months posttransplantation. The lymphocyte subsets including CD56+/CD3− natural killer (NK) cells and T-cell subsets including CD4+, CD8+, and regulatory T cells (CD4+/CD25bright/CD127low) were measured by flow cytometry using established methods.17
Study objectives
The primary objective of our study was to determine the association between pretransplant iron overload and 1-year overall survival (OS). Secondary objectives included cumulative incidence of a composite end point of nonrelapse mortality (NRM) and complications (serious infections, hepatic veno-occlusive disease [VOD], or organ failure) at 1 year, cumulative incidence of acute or chronic graft-versus-host-disease (GVHD), prevalence of pretransplant and 1-year posttransplant iron overload, and correlation between various measures of iron overload and assessment of post-HCT immune reconstitution. For the outcome end points, we assessed iron overload using an LIC threshold of >1.8 mg/g as well as a cutoff of >5 mg/g (clinically significant iron overload).4,10,11
Study definitions
Disease risk at HCT was classified as standard or high risk. Acute leukemia in first or second complete remission, chronic myeloid leukemia (CML) in first chronic phase, lymphoma in complete remission or chemotherapy-sensitive partial remission, and myelodysplastic syndromes (MDS; refractory anemia or refractory anemia with ringed sideroblasts subtypes) were considered standard risk; all other diagnoses were classified as high risk. Patients were assigned HCT comorbidity index scores pretransplantation according to standard criteria.18
OS was defined as death from any cause. NRM was defined as death from any cause other than relapse or disease progression. Infections and organ failure were defined a priori. Serious bacterial infections included clinically defined infections, such as pneumonia or cellulitis, as well as organism-identified cultures from sputum, bronchial, biliary, blood, urinary, or cerebral spinal fluid specimens that needed treatment with antibiotics. Viral infections were defined as detectable levels of virus requiring therapy, including asymptomatic cytomegalovirus (CMV) reactivation. Fungal infections included localized, invasive, and disseminated infections requiring therapy. Respiratory failure was defined as need for mechanical ventilation or noninvasive modes of ventilation (eg, bilevel positive airway pressure ventilation) outside of an elective procedure. Cardiac failure was established as the need for inotropic support and/or a clinically significant reduction in left ventricular ejection fraction compared with pre-HCT baseline. Renal failure was defined as the need for dialysis. Standard criteria were used for the diagnosis and staging of hepatic VOD and acute and chronic GVHD.19-21
Statistical analysis
Patient and transplant characteristics were compared using the χ2 test, Fisher’s exact test, or Wilcoxon test, as applicable. OS was estimated using Kaplan-Meier curves and survival curves were compared using the log-rank test.22 Cumulative incidence estimates were reported for relapse, NRM, GVHD, organ failure, VOD, and infections.23 For GVHD, relapse, organ failure, VOD, and infection, death without the event was treated as the competing risk. For NRM, relapse was the competing risk.
The independent effect of iron overload on OS was evaluated using Cox regression.24 Models were adjusted for conditioning intensity, donor type, ABO match, diagnosis, CMV serostatus, HCT comorbidity index score, patient age, and disease risk. Fine and Gray regression was used to assess the independent effect of iron overload on NRM and our composite end point of NRM and complications.25
Repeated measures of ferritin over time (pre-HCT to 12 months) were evaluated using generalized linear mixed models treating the intercept as a random factor and taking the different variances of pretransplant iron overload into consideration. The estimation of the variance for ferritin was significantly higher in the iron-overload group than in the no iron-overload group. An unstructured variance/covariance was assumed.26 Mixed models were also used to evaluate the effect of baseline iron overload on the repeated measures of immune reconstitution over the span of 1 year. These measures included the absolute lymphocyte count and absolute counts of NK cells, CD4+ T cells, CD8+ T cells, and regulatory-phenotype T cells.
Results
Patient characteristics
Our study enrolled 112 patients, and 24 were excluded for the following reasons: 12 did not proceed to transplant because of disease progression, 9 were unable to complete pretransplant MRI, and 3 encountered significant delays between enrollment and date of transplantation. Of the 88 patients remaining in the study, 60 (68%) had iron overload and 28 (32%) did not. Four patients with ferritin >500 ng/mL who were found to have LIC ≤1.8 mg/g were considered as part of the no iron-overload group.
Table 1 summarizes patient demographics and transplant characteristics. Male sex and high-risk disease were significantly more common among the no iron-overload cohort. A greater proportion of patients with iron overload were CMV seropositive.
Patient demographics and transplant characteristics
| Characteristics . | No iron overload . | Iron overload . | P . |
|---|---|---|---|
| N | 28 | 60 | |
| Median age (range), y | 52 (19-70) | 52 (20-69) | .85 |
| Male gender | 23 (82%) | 37 (62%) | .05 |
| HCT comorbidity index | .12 | ||
| 0 | 17 (61%) | 30 (50%) | |
| 1-2 | 3 (11%) | 14 (23%) | |
| ≥3 | 5 (18%) | 15 (25%) | |
| Missing | 3 (10%) | 1 (2%) | |
| Diagnosis | <.01 | ||
| ALL | 1 (4%) | 9 (15%) | |
| AML | 3 (11%) | 24 (40%) | |
| MDS | 4 (14%) | 11 (18%) | |
| Lymphoma | 7 (25%) | 12 (20%) | |
| Multiple myeloma | 7 (25%) | 1 (2%) | |
| Other | 6 (21%) | 3 (5%) | |
| High disease risk | 21 (75%) | 24 (40%) | <.01 |
| Conditioning regimen | .46 | ||
| Myeloablative | 5 (18%) | 15 (25%) | |
| Reduced intensity | 23 (82%) | 45 (75%) | |
| Graft source | .11 | ||
| Marrow | 5 (18%) | 5 (8%) | |
| PBSCs | 14 (50%) | 22 (37%) | |
| UCB | 9 (32%) | 33 (55%) | |
| Donor type | .11 | ||
| Matched sibling | 14 (50%) | 20 (33%) | |
| Matched unrelated | 5 (18%) | 6 (10%) | |
| Mismatched unrelated | 0 | 1 (2%) | |
| Single UCB | 0 | 1 (2%) | |
| Double UCB | 9 (32%) | 32 (53%) | |
| Gender-mismatched graft | 18 (64%) | 39 (65%) | .95 |
| ABO blood match | .79 | ||
| Match | 12 (43%) | 24 (40%) | |
| Minor mismatch | 6 (21%) | 10 (17%) | |
| Major mismatch | 9 (32%) | 25 (42%) | |
| CMV serostatus | .03 | ||
| Patient−/donor− | 12 (43%) | 15 (25%) | |
| Patient−/donor+ | 5 (18%) | 4 (7%) | |
| Patient+ | 11 (39%) | 41 (68%) |
| Characteristics . | No iron overload . | Iron overload . | P . |
|---|---|---|---|
| N | 28 | 60 | |
| Median age (range), y | 52 (19-70) | 52 (20-69) | .85 |
| Male gender | 23 (82%) | 37 (62%) | .05 |
| HCT comorbidity index | .12 | ||
| 0 | 17 (61%) | 30 (50%) | |
| 1-2 | 3 (11%) | 14 (23%) | |
| ≥3 | 5 (18%) | 15 (25%) | |
| Missing | 3 (10%) | 1 (2%) | |
| Diagnosis | <.01 | ||
| ALL | 1 (4%) | 9 (15%) | |
| AML | 3 (11%) | 24 (40%) | |
| MDS | 4 (14%) | 11 (18%) | |
| Lymphoma | 7 (25%) | 12 (20%) | |
| Multiple myeloma | 7 (25%) | 1 (2%) | |
| Other | 6 (21%) | 3 (5%) | |
| High disease risk | 21 (75%) | 24 (40%) | <.01 |
| Conditioning regimen | .46 | ||
| Myeloablative | 5 (18%) | 15 (25%) | |
| Reduced intensity | 23 (82%) | 45 (75%) | |
| Graft source | .11 | ||
| Marrow | 5 (18%) | 5 (8%) | |
| PBSCs | 14 (50%) | 22 (37%) | |
| UCB | 9 (32%) | 33 (55%) | |
| Donor type | .11 | ||
| Matched sibling | 14 (50%) | 20 (33%) | |
| Matched unrelated | 5 (18%) | 6 (10%) | |
| Mismatched unrelated | 0 | 1 (2%) | |
| Single UCB | 0 | 1 (2%) | |
| Double UCB | 9 (32%) | 32 (53%) | |
| Gender-mismatched graft | 18 (64%) | 39 (65%) | .95 |
| ABO blood match | .79 | ||
| Match | 12 (43%) | 24 (40%) | |
| Minor mismatch | 6 (21%) | 10 (17%) | |
| Major mismatch | 9 (32%) | 25 (42%) | |
| CMV serostatus | .03 | ||
| Patient−/donor− | 12 (43%) | 15 (25%) | |
| Patient−/donor+ | 5 (18%) | 4 (7%) | |
| Patient+ | 11 (39%) | 41 (68%) |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; PBSCs, peripheral blood stem cells; UCB, umbilical cord blood.
Twenty-one patients died during the 1-year study follow-up period (iron overload = 16 [27%], no iron overload = 5 [18%]). Seven patients withdrew from the study and did not complete 1-year serum ferritin assessments (iron overload = 5 [8%], no iron overload = 2 [7%]).
The median number of red blood cell (RBC) transfusions during the 12-month follow-up period was 6 (interquartile range [IQR], 2-11) for patients with no iron overload and 10 (IQR, 4-24) for those with iron overload (P < .01). Information on RBC transfusions prior to transplantation was available for a limited number of patients. These data were available for 10 (36%) patients with no iron overload (median units transfused = 3 [IQR, 0-8]) and 35 (58%) patients with iron overload (median units transfused = 8 [IQR, 2-19]).
Pre- and Post-HCT measures of iron overload
Median pretransplant ferritin levels in patients with and without iron overload were 1731.5 ng/mL (range, 510-7137 ng/mL) and 289.5 ng/mL (range, 52-2023 ng/mL), respectively. Among patients with iron overload, median LIC was 4.3 mg/g (range, 1.9-25.4 mg/g). The prevalence of pre-HCT iron overload (LIC >1.8 mg/g) was 68% (95% confidence interval [CI], 58%-78%) and the prevalence of clinically significant iron overload (LIC >5 mg/g) was 26% (95% CI, 17%-35%). Using data from the 64 patients who received both measurements, we observed a modest correlation between serum ferritin and LIC (Spearman R = 0.58 [95% CI, 0.46-0.70]; Figure 1A). Correlation did not improve with higher cutoffs for ferritin (Spearman R = 0.55 for ferritin >1000 ng/mL and 0.42 for ferritin >2000 ng/mL). We used recursive partitioning analysis to define the best cut point for baseline ferritin for prediction of iron overload. Pre-HCT ferritin ≥496 ng/mL best predicted LIC >1.8 mg/g in our cohort, and ferritin ≥1754 ng/mL was the best cut point for predicting LIC >5 mg/g.
Correlation between and changes in ferritin and LIC in HCT recipients. (A) Scatterplot showing the relationship of pretransplant ferritin level with R2-MRI–measured liver iron content; (B) box plots showing change in ferritin level over time among patients without and with pretransplant iron overload; (C) scatterplot showing relationship of ferritin level with R2-MRI–measured liver iron content at 12 months posttransplantation; and (D) change in liver iron content from pretransplant to 12 months posttransplantation (dark line represents trend for all observations).
Correlation between and changes in ferritin and LIC in HCT recipients. (A) Scatterplot showing the relationship of pretransplant ferritin level with R2-MRI–measured liver iron content; (B) box plots showing change in ferritin level over time among patients without and with pretransplant iron overload; (C) scatterplot showing relationship of ferritin level with R2-MRI–measured liver iron content at 12 months posttransplantation; and (D) change in liver iron content from pretransplant to 12 months posttransplantation (dark line represents trend for all observations).
Serial ferritin measurements at 3, 6, 9, and 12 months posttransplant remained significantly elevated in the iron-overload cohort compared with the no iron-overload cohort (P < .01; Figure 1B). We observed a modest correlation between serum ferritin and LIC at 12 months post-HCT (Spearman R = 0.72 [95% CI, 0.57-0.87]; Figure 1C). Among patients with pre-HCT iron overload, the mean change in LIC from pre-HCT to 12 months post-HCT was 2.1 mg/g (standard deviation = 5.5 mg/g; Figure 1D).
Among patients with pre-HCT and 12-month post-HCT ferritin and LIC information, we reviewed the use of RBC transfusions during the 12-month follow-up period and evaluated their correlation with change in ferritin and change in LIC. The correlation between the number of RBC transfusions during this time period and change in ferritin was modest (Spearman R = 0.40 [95% CI, 0.03-0.66]) and change in LIC was poor (Spearman R = 0.25 [95% CI, 0.12-0.56]).
Association of iron overload and outcomes
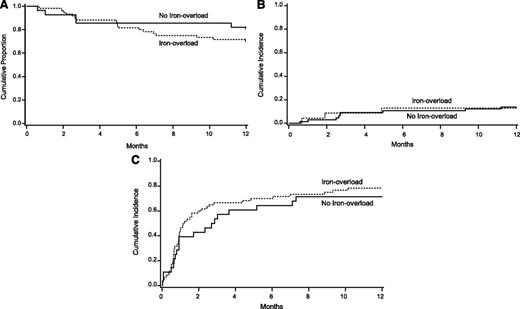
Table 2 shows results of univariate analyses of the association of iron overload with our study outcomes. We observed no significant difference in 1-year OS between the iron-overload and no iron-overload cohorts (Figure 2). Similarly, we found no association between iron-overload status and our secondary end points of NRM, relapse, infections, organ failure, VOD, or GVHD or the composite end point of NRM and complications. In univariate analyses using an iron-overload definition of LIC >5 mg/g, we again did not find any association between iron overload and our primary and secondary end points.
Iron overload status and univariate outcomes
| Outcome . | Iron overload defined as LIC >1.8 mg/g . | P . | Iron overload defined as LIC >5 mg/g . | P . | ||
|---|---|---|---|---|---|---|
| No iron overload, Prob (95% CI) . | Iron overload, Prob (95% CI) . | No iron overload, Prob (95% CI) . | Iron overload, Prob (95% CI) . | |||
| N | 28 | 60 | 65 | 23 | ||
| Overall survival, 1 y | 82% (62-92) | 72% (58-81) | .33 | 74% (61-83) | 78% (55-90) | .68 |
| NRM, 1 y* | 14% (2%-27%) | 13% (5%-22%) | .86 | 14% (5%-22%) | 13% (0%-26%) | .95 |
| Relapse, 1 y* | 21% (6%-73%) | 23% (13%-34%) | .92 | 22% (9%-36%) | 23% (12%-35%) | .91 |
| Composite end point, 1 y*† | 71% (51%-91%) | 80% (65%-95%) | .44 | 72% (53%-91%) | 81% (55%-97%) | .94 |
| Serious infections, 1 y* | 64% (43%-85%) | 73% (59%-88%) | .35 | 69% (55%-83%) | 74% (51%-97%) | .53 |
| Bacterial infections, 1 y* | 11% (0%-22%) | 13% (5%-22%) | .72 | 12% (4%-20%) | 13% (0%-27%) | .98 |
| Viral infections, 1 y* | 57% (37%-77%) | 68% (54%-83%) | .27 | 63% (49%-77%) | 70% (46%-93%) | .48 |
| Fungal infections, 1 y* | 7% (0%-17%) | 12% (4%-20%) | .49 | 12% (4%-20%) | 4% (0%-12%) | .28 |
| Respiratory failure, 1 y* | 11% (0%-22%) | 18% (9%-27%) | .38 | 17% (8%-26%) | 13% (1%-26%) | .64 |
| Cardiac failure, 1 y* | 0% | 8% (1%-15%) | .13 | 6% (1%-12%) | 9% (0%-20%) | .53 |
| Renal failure, 1 y* | 7% (0%-16%) | 10% (3%-17%) | .66 | 9% (2%-16%) | 9% (0%-20%) | .92 |
| Hepatic VOD, 1 y* | 4% (1%-8%) | 0% | .32 | 2% (0%-5%) | 0% | .74 |
| Acute GVHD, 100 days* | 50% (30%-70%) | 33% (21%-45%) | .16 | 42% (29%-54%) | 30% (12%-49%) | .44 |
| Chronic GVHD, 1 y* | 21% (6%-37%) | 17% (7%-26%) | .72 | 22% (9%-36%) | 15% (6%-25%) | .55 |
| Outcome . | Iron overload defined as LIC >1.8 mg/g . | P . | Iron overload defined as LIC >5 mg/g . | P . | ||
|---|---|---|---|---|---|---|
| No iron overload, Prob (95% CI) . | Iron overload, Prob (95% CI) . | No iron overload, Prob (95% CI) . | Iron overload, Prob (95% CI) . | |||
| N | 28 | 60 | 65 | 23 | ||
| Overall survival, 1 y | 82% (62-92) | 72% (58-81) | .33 | 74% (61-83) | 78% (55-90) | .68 |
| NRM, 1 y* | 14% (2%-27%) | 13% (5%-22%) | .86 | 14% (5%-22%) | 13% (0%-26%) | .95 |
| Relapse, 1 y* | 21% (6%-73%) | 23% (13%-34%) | .92 | 22% (9%-36%) | 23% (12%-35%) | .91 |
| Composite end point, 1 y*† | 71% (51%-91%) | 80% (65%-95%) | .44 | 72% (53%-91%) | 81% (55%-97%) | .94 |
| Serious infections, 1 y* | 64% (43%-85%) | 73% (59%-88%) | .35 | 69% (55%-83%) | 74% (51%-97%) | .53 |
| Bacterial infections, 1 y* | 11% (0%-22%) | 13% (5%-22%) | .72 | 12% (4%-20%) | 13% (0%-27%) | .98 |
| Viral infections, 1 y* | 57% (37%-77%) | 68% (54%-83%) | .27 | 63% (49%-77%) | 70% (46%-93%) | .48 |
| Fungal infections, 1 y* | 7% (0%-17%) | 12% (4%-20%) | .49 | 12% (4%-20%) | 4% (0%-12%) | .28 |
| Respiratory failure, 1 y* | 11% (0%-22%) | 18% (9%-27%) | .38 | 17% (8%-26%) | 13% (1%-26%) | .64 |
| Cardiac failure, 1 y* | 0% | 8% (1%-15%) | .13 | 6% (1%-12%) | 9% (0%-20%) | .53 |
| Renal failure, 1 y* | 7% (0%-16%) | 10% (3%-17%) | .66 | 9% (2%-16%) | 9% (0%-20%) | .92 |
| Hepatic VOD, 1 y* | 4% (1%-8%) | 0% | .32 | 2% (0%-5%) | 0% | .74 |
| Acute GVHD, 100 days* | 50% (30%-70%) | 33% (21%-45%) | .16 | 42% (29%-54%) | 30% (12%-49%) | .44 |
| Chronic GVHD, 1 y* | 21% (6%-37%) | 17% (7%-26%) | .72 | 22% (9%-36%) | 15% (6%-25%) | .55 |
Prob, probability.
Cumulative incidence estimate.
Composite end point includes NRM and complications (serious infections, hepatic VOD, or organ failure).
Outcomes by iron-overload status. (A) Overall survival, (B) cumulative incidence of NRM, and (C) composite end point of NRM and complications.
Outcomes by iron-overload status. (A) Overall survival, (B) cumulative incidence of NRM, and (C) composite end point of NRM and complications.
In multivariate analyses, we did not observe a significant association between iron overload and survival (relative risk [RR] = 2.3; 95% CI, 0.9-5.9; P = .08 compared with no iron overload). Similarly, iron overload had no independent impact on NRM (RR = 2.0; 95% CI, 0.6-6.9; P = .26) or our composite end point of NRM and complications (RR = 0.8; 95% CI, 0.4-1.4; P = .41). We also conducted analyses using a higher LIC cutoff (>5 mg/g) to define iron overload and again found no association of iron overload with survival (RR = 0.8; 95% CI, 0.3-2.0; P = .58 compared with no iron overload).
We also conducted multivariate analyses to evaluate the association of ferritin with outcomes. Ferritin >1000 ng/mL was an independent predictor of overall survival (RR = 2.4; 95% CI, 1.0-5.6; P = .05) and of NRM (RR = 4.2; 95% CI, 1.1-15.7; P = .03). However, ferritin was not found to be associated with our composite end point of NRM and complications (RR = 1.1; 95% CI, 0.7-1.9; P = .64).
Iron overload and immune reconstitution
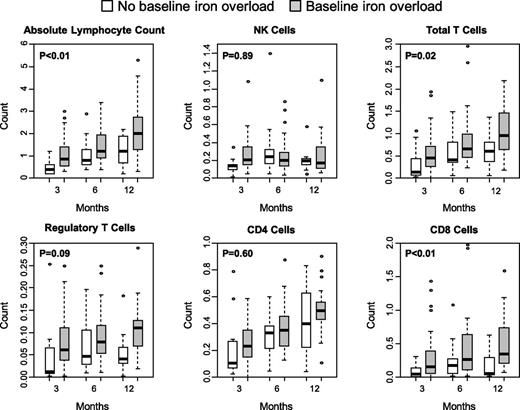
Immune reconstitution studies were performed in 55 patients at 3, 6, and 12 months post-HCT (Figure 3). Iron overload was not associated with delay in recovery of absolute lymphocyte count, absolute NK cells, total T cells, and CD4+, CD8+, or regulatory phenotype T cells. In generalized linear mixed models that examined repeated measures of immune reconstitution, patients with iron overload had significantly higher absolute lymphocyte counts, total T cells, and CD8+ T cells over the 12-month follow-up period.
Iron-overload status and immune reconstitution parameters at 3, 6, and 12 months posttransplantation. Counts are in cells per μL.
Iron-overload status and immune reconstitution parameters at 3, 6, and 12 months posttransplantation. Counts are in cells per μL.
Discussion
In our prospective cohort study, we found no association between iron overload, defined using R2-MRI–measured LIC levels, and OS, NRM, or complications among allogeneic HCT recipients. Our findings are in contrast to other publications that have used serum ferritin level as a surrogate measure of iron overload and have found an association between high ferritin levels and mortality after allogeneic HCT.1-3 We also found an association between elevated ferritin and overall survival and NRM; however, high LIC was not predictive of either outcome. LIC is a more accurate measure of body iron stores compared with ferritin, because ferritin loses its specificity for iron overload in the setting of underlying infections or inflammation, conditions not uncommon among patients undergoing allogeneic transplantation.
Literature on the association of LIC with allogeneic HCT outcomes is sparse, although 2 studies have been recently reported.11,27 In the first study, Armand et al prospectively observed 45 patients with MDS or acute leukemia undergoing myeloablative HCT over 1 year posttransplantation and conducted serial measurements of serum iron parameters and liver and cardiac MRI scans.11 Pre-HCT LIC was found to have no association with mortality, relapse, or GVHD, although ferritin was observed to have prognostic significance as previously reported in the literature. Wermke et al have reported a study of 88 patients with MDS and acute leukemia who underwent MRI prior to allogeneic HCT to measure LIC.27 LIC ≥125 µmol/g was significantly associated with higher risks of overall mortality (hazard ratio = 2.24) and NRM (hazard ratio = 2.98). However, serum ferritin was found to have no impact on transplant outcomes on multivariate analyses. Taken together, our and the 2 previously reported studies provide valuable insight into our understanding of iron overload and allogeneic HCT outcomes. Most importantly, the jury is still out whether pre-HCT iron overload has any influence on survival, NRM, and other complications following allogeneic HCT. Studies based on serum ferritin, which have predominantly shown that hyperferritinemia is associated with overall mortality and NRM, have to be interpreted with caution. Some providers have adopted routine chelation therapy for patients with high ferritin pre-HCT; this practice needs to be reevaluated until stronger evidence for its use is available, given its costs, toxicity, and unclear benefit. All 3 studies were limited by a relatively small sample size, and more large studies that use LIC instead of ferritin-defined end points are still needed to evaluate the association of iron overload with allogeneic HCT outcomes.
Small retrospective case series have reported the possible association of iron overload with infections, GVHD, hepatic VOD, and hepatic dysfunction in allogeneic HCT recipients.4,28-32 In our study, with a priori–identified end points and prospective patient follow-up, we found no association of iron overload with HCT complications including infections, organ failure, VOD, or GVHD. Two other studies described above that have used LIC to define iron overload also reported no association of iron overload with infections, VOD, or GVHD.11,27 Again, our findings should reassure clinicians that it is acceptable to avoid chelation therapy in patients with pretransplant iron overload while more research is conducted in this area. This is especially relevant because chelation therapy is not without risk of toxicity (eg, nephrotoxicity with deferasirox, hepatotoxicity with deferiprone, and ototoxicity with deferoxamine).
Our study provides important insights into the evaluation and natural history of iron overload in allogeneic HCT recipients. First, a substantial number of patients have iron overload at the time of transplantation. In our cohort, 68% had iron overload and 26% had clinically significant iron overload (LIC ≥5 mg/g). We also confirm that ferritin is a sensitive but not a specific measure of iron overload among allogeneic HCT recipients. Furthermore, and as reported by other studies,11,27 its correlation with LIC is modest and the degree of ferritin elevation does not accurately predict body iron stores. Hence, research studies and, where indicated, clinical assessment of iron overload in allogeneic HCT recipients should be based on LIC measurements instead of ferritin. We and Armand et al found poor correlation between RBC transfusion history after transplantation and change in ferritin or LIC over the 12-month follow-up period.11 There can be several factors that can lead to decrease in body iron stores posttransplantation (eg, red cell loss through phlebotomy/bleeding or iron utilization for erythropoiesis).4,15 Hence, treatment decisions for iron overload in allogeneic HCT survivors should not be based on transfusion history alone and assessment of LIC should be considered in these patients.
The role of excess iron in the recovery of immunity posttransplantation has not been described previously. However, iron has been shown to be important in innate and adaptive immune responses in patients with hereditary hemochromatosis and nontransplant transfusional iron overload. Iron overload can lead to defective chemotaxis and phagocytosis by neutrophils and macrophages, decreased NK cell activity, and a decrease in the number and altered function of CD4+ and CD8+ T cells.33,34 We did not observe any association of iron overload with delay in the recovery of several lymphocyte subsets including T cells and NK cells. However, our findings are not definitive and primarily lay the foundation for more work in this area.
Some limitations of our study have to be considered. First, our sample size was relatively small and larger studies are still needed to evaluate the association of iron overload with allogeneic HCT outcomes. Because of this limitation, we were not able to conduct subgroup analyses using higher LIC thresholds for defining iron overload, which might be more likely to have an association with transplant mortality and complications. Our study population was heterogeneous because we included all diagnoses and conditioning regimens. Patients with ferritin levels <500 ng/mL did not undergo MRI assessments to measure LIC; however, it is very unlikely that these patients had iron overload because ferritin is a highly sensitive test for iron overload. Because we designed our study to follow-up patients through 1 year posttransplantation, we cannot address the impact of iron overload on long-term survival after allogeneic HCT. The role of iron overload in the pathogenesis of late complications, especially liver and cardiac dysfunction, and whether treatment of iron overload with chelation or phlebotomy reduces their risks need to be investigated.
There has been an increasing interest in using iron chelation in the peritransplant period in a nonclinical trial setting among patients with high pre-HCT ferritin levels. Our findings suggest caution with this approach and highlight the need for continued research on the role of iron overload in allogeneic HCT recipients.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jenna Johnson and Roberta Nicklow for assistance with conducting this study and Julie Curtsinger for help with immune reconstitution studies.
This work was supported in part by a grant from the National Institutes of Health (P30CA77598) utilizing the following Masonic Cancer Center, University of Minnesota shared resources: Clinical Trials Office, Oncology Medical Informatics and Services, and Translational Cell Therapy. Research funding for this study was provided by Novartis Pharmaceuticals Corporation, East Hanover, NJ.
Authorship
Contribution: N.M. had full access to all of the data in the study and had final responsibility for the integrity of the data, the accuracy of the data analysis, and the responsibility for the decision to submit for publication; L.B., T.D., S.C., and N.M. designed research; N.M. obtained funding; B.T., T.D., S.C., and N.M. collected data; T.D. and N.M. performed statistical analysis; B.T., L.B., and N.M. drafted the manuscript; and all authors interpreted data, critically revised the manuscript, and reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: N.M. received research funding from Novartis Pharmaceuticals Corporation for conducting this study. The remaining authors declare no competing financial interests.
Correspondence: Navneet Majhail, National Marrow Donor Program, 3001 Broadway St NE, Suite 100, Minneapolis, MN 55413; e-mail: nmajhail@nmdp.org.