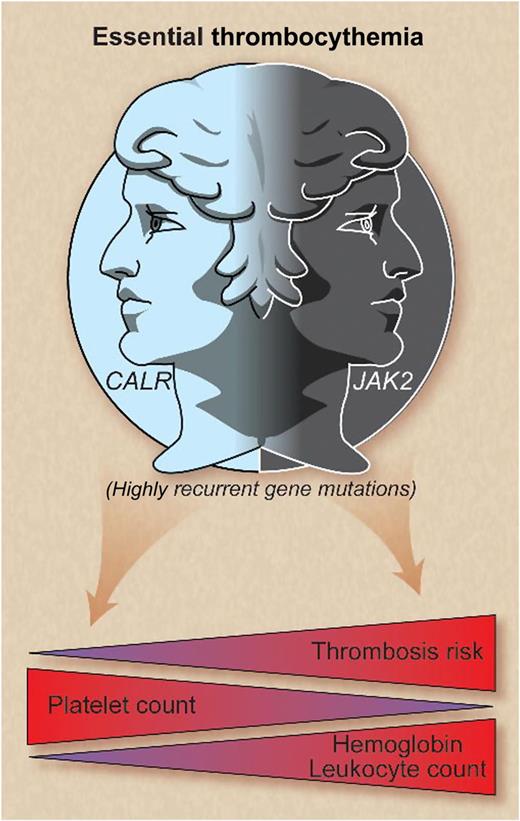
In this issue of Blood, Rumi et al and Rotunno et al demonstrate that essential thrombocythemia (ET) patients with calreticulin mutations exhibit lower leukocyte and hemoglobin values, higher platelet counts, and a lower thrombosis risk vs JAK2-mutated ET. Calreticulin-mutated ET appears to be a distinct entity with a more indolent course.1,2
CALR and JAK2 mutations represent 2 disease spectrums in essential thrombocythemia whereby cases with mutated CALR are characterized by higher platelet levels, lower hemoglobin and leukocyte counts, and lower thrombosis risk compared with JAK2-mutated patients. Professional illustration by Debra T. Dartez.
CALR and JAK2 mutations represent 2 disease spectrums in essential thrombocythemia whereby cases with mutated CALR are characterized by higher platelet levels, lower hemoglobin and leukocyte counts, and lower thrombosis risk compared with JAK2-mutated patients. Professional illustration by Debra T. Dartez.
Calreticulin (CALR) is a highly conserved multifunctional endoplasmic reticulum (ER) protein. Inside the ER, CALR plays an integral role in calcium homeostasis and protein folding. Outside the ER, CALR is found in the cytosol and on the cell surface, where it regulates integrin-mediated cell adhesion, gene nuclear transport, programmed cell removal, and immunogenic cell death. The CALR protein structure contains 3 distinct domains with specific functions. The N- and P-domains are mainly responsible for the protein’s chaperone function, whereas the C-domain is principally involved in calcium regulation in the ER.3 The C-domain also contains the KDEL sequence, which is responsible for preventing secretion of the protein from the ER.
Recently, recurrent CALR indel mutations were discovered in ET and primary myelofibrosis (PMF) patients with nonmutated JAK2 and MPL.4,5 All CALR mutations were localized to exon 9 and uniformly led to loss of most of the C-terminal acidic domain, multiple calcium-binding sites, and the KDEL sequence. These mutations were an early event in myeloproliferative neoplasm (MPN) tumorigenesis.5
CALR has been implicated in human cancers by playing roles in macrophage-mediated immune evasion,6 activation of the unfolded protein response (UPR),7 and calcium signaling. The consequence of these CALR mutations in MPNs is unknown but may be related to one or more of the aforementioned functions. First, CALR mutations may lead to evasion of macrophage phagocytosis. We demonstrated that many cancers constitutively express cell surface CALR and that increased CALR mRNA expression is associated with a poor prognosis.6 Cell surface CALR serves as a pro-phagocytic signal that facilitates clearance of damaged cells.8 Although one might expect increased CALR cell surface expression to lead to increased cell removal due to its pro-phagocytic function, this effect is counterbalanced by overexpression of the antiphagocytic signal CD47. In MPNs, it is possible that CALR mutations, due to loss of the KDEL ER retention sequence, disrupt CALR cell surface expression, leading to decreased susceptibility to phagocytosis. Although initial reports of the CALR-mutated MPN patients are mixed in demonstrating abnormal localization of CALR in the cytosol4 /cell membrane,5 further investigation is needed. Second, CALR mutations may cause disruption of the UPR, leading to a competitive growth advantage. In response to ER stress, the UPR is activated to prevent abnormal cell proliferation by stopping protein translation through activation of ER protein folding chaperones. Indeed, perturbation of the UPR has been implicated in human cancers.9 Mutations may impair CALR protein chaperone function in the ER and disrupt the homeostatic UPR response, leading to cell hyperproliferation. Last, it is very likely that CALR mutations play a role in altered signal transducer and activator of transcription (STAT) signaling, similar to JAK2 V617F. This hypothesis is supported by several observations. First, CALR mutations are mutually exclusive to JAK2 and MPL mutations in ET and PMF patients,4,5 suggesting a redundant role of these genes. Second, increased STAT5 phosphorylation was observed in CALR mutant transfected cell lines compared with wild type.4 Third, the Janus kinase (JAK) inhibitor ruxolitinib elicited responses in CALR-mutated, hydroxyurea-refractory ET patients, similar to those observed in JAK2-mutated patients.1
To establish the clinical implications of these molecular findings, Rotunno et al compared mutational subgroups of ET,2 and Rumi and colleagues compared cohorts of CALR- and JAK2-mutated ET to patients with polycythemia vera (PV).1 Compared with JAK2-mutated ET, both groups found that CALR-mutated ET patients were more commonly male, demonstrated higher platelet counts, and lower leukocyte and hemoglobin levels (see figure).1,2 Individuals with PV exhibited higher leukocyte and hemoglobin values compared with CALR- and JAK2-mutated ET patients but lower platelet counts and serum erythropoietin levels.1
Both analyses identified a lower incidence of thrombosis in CALR vs JAK2-mutated ET patients (see figure),1,2 but thrombosis rates were not different between PV and JAK2-mutated ET.1 Rotunno et al identified a 10-year cumulative incidence of thrombosis of 5.1% vs 14.5% in CALR- vs JAK2-mutated patients, respectively,2 paralleling the 15-year cumulative thrombosis incidence of 10.5% vs 25.1% identified by Rumi et al.1 The higher thrombosis rate in JAK2-mutated ET patients may be partly attributable to the hyperviscosity associated with higher hematocrit and leukocyte levels, the latter being a putative risk factor for thrombosis in MPNs. Evaluation of leukocyte and platelet activation, as well as hypercoagulability profiles, will lend further insight into this differential propensity for thrombosis.
The effect of mutational status on the clinical course of ET was evaluated in both studies and was also compared with PV. Although ET patients carrying a CALR mutation exhibited a better overall survival than PV patients, a nonsignificant trend1 or no difference in survival2 was found between CALR and JAK2-mutated ET patients. In contrast, a significant difference (P = .04) was observed in 10-year overall survival between these ET subgroups by Klampfl et al.4 The 15-year cumulative incidence of secondary myelofibrosis was similar between CALR- and JAK2-mutated ET and PV,1 but evolution to leukemia was lower for CALR-mutated ET patients compared with the other groups.1 Whereas a significant fraction of JAK2-mutated ET patients progressed to PV, none with a CALR mutation did so.1 Consistent with prior data, the median JAK2 mutant allele burden was higher in ET patients who progressed to PV, as well as in post-PV/ET MF compared with the initial MPN.1 These data reinforce that PV and JAK2-mutated ET form a continuum wherein phenotype and natural history are substantially influenced by JAK2 allele burden.
While investigators decipher how CALR mutations contribute to MPN pathogenesis, it is clear that CALR will be quickly assimilated into World Health Organization diagnostic criteria for ET and PMF. Less clear is whether CALR mutation status will provide independent prognostic utility in scoring systems10 used to estimate vascular risk and survival. Also, the effect of CALR mutant allele burden on clinical correlates remains untested. Looking forward, these observational studies provide a framework for assessing whether conventional therapies used broadly for ET (eg, aspirin for low-risk patients and hydroxyurea for high-risk individuals) are also appropriate for CALR-mutated patients who may exhibit lower-risk features.
Conflict-of-interest disclosure: J.G. receives funding for administration of clinical trials, serves on an advisory board, and receives honoraria from Incyte, Inc. The remaining author declares no competing financial interests.