Key Points
In β-thalassemia major patients with severe iron burden, deferasirox was noninferior to deferoxamine for myocardial iron removal.
The ejection fraction was stable during treatment for both deferasirox and deferoxamine.
Abstract
Randomized comparison data on the efficacy and safety of deferasirox for myocardial iron removal in transfusion dependent patients are lacking. CORDELIA was a prospective, randomized comparison of deferasirox (target dose 40 mg/kg per day) vs subcutaneous deferoxamine (50-60 mg/kg per day for 5-7 days/week) for myocardial iron removal in 197 β-thalassemia major patients with myocardial siderosis (T2* 6-20 milliseconds) and no signs of cardiac dysfunction (mean age, 19.8 years). Primary objective was to demonstrate noninferiority of deferasirox for myocardial iron removal, assessed by changes in myocardial T2* after 1 year using a per-protocol analysis. Geometric mean (Gmean) myocardial T2* improved with deferasirox from 11.2 milliseconds at baseline to 12.6 milliseconds at 1 year (Gmeans ratio, 1.12) and with deferoxamine (11.6 milliseconds to 12.3 milliseconds; Gmeans ratio, 1.07). The between-arm Gmeans ratio was 1.056 (95% confidence interval [CI], 0.998, 1.133). The lower 95% CI boundary was greater than the prespecified margin of 0.9, establishing noninferiority of deferasirox vs deferoxamine (P = .057 for superiority of deferasirox). Left ventricular ejection fraction remained stable in both arms. Frequency of drug-related adverse events was comparable between deferasirox (35.4%) and deferoxamine (30.8%). CORDELIA demonstrated the noninferiority of deferasirox compared with deferoxamine for myocardial iron removal. This trial is registered at www.clinicaltrials.gov as #NCT00600938.
Introduction
Without effective iron chelation therapy, patients with transfusional iron overload are at risk of iron deposition in vital organs such as the liver and heart. The heart is more sensitive to iron overload than the liver, whereby lower levels of iron are sufficient to cause iron-related heart failure and death, relative to the larger iron load that can be tolerated by the liver before hepatic dysfunction occurs.1 Significant variation has been observed between heart and liver iron loading,2 which is incompletely understood. Iron may load earlier in the liver and only later in the myocardium, with differential kinetics of iron chelators between organs playing a role.3 In β-thalassemia major patients treated with deferoxamine, evidence of myocardial iron deposition only becomes apparent toward the end of the first decade of life.4
Although a decrease in cardiac-related mortality has recently become apparent,5 heart failure due to iron-induced cardiomyopathy remains a key cause of death in patients with β-thalassemia major.6-9 The decrease in cardiac-related mortality is due in part to the introduction of T2* cardiovascular magnetic resonance (CMR) for the estimation of myocardial iron, which has contributed to improved management of cardiac siderosis.10-15 T2* CMR is now widely used, is highly reproducible,16-18 and is calibrated in animal19 and human hearts.1 Myocardial T2* >20 milliseconds is considered normal2 and iron accumulation causes a reduction in T2*, with values <10 milliseconds being associated with increased risk of heart failure.15
Iron chelation therapy aims to prevent iron accumulation or to remove iron deposition when it has already occurred. The available iron chelators DFO, deferiprone, and deferasirox can all remove myocardial iron with acceptable safety profiles.14,20-25 However, there are limited data from randomized controlled trials comparing iron chelation therapies for removal of myocardial iron14,22,25 and none for deferasirox. Efficacy and safety of deferasirox in myocardial iron removal has only been reported in uncontrolled single-arm trials.20,26-28 Here, we describe the first prospective, randomized comparison of changes in myocardial T2* with deferasirox or DFO in β-thalassemia major patients with myocardial siderosis. The primary objective was to demonstrate noninferiority of deferasirox when compared with DFO in myocardial iron removal, as assessed by changes in myocardial T2* after 1 year.
Methods
Patients
CORDELIA was conducted between April 10, 2008, and March 1, 2012. Patients with β-thalassemia major, Diamond–Blackfan anemia, low/intermediate 1 myelodysplastic syndromes, or sideroblastic anemia, aged ≥10 years with myocardial T2* 6 to 20 milliseconds without clinical symptoms of cardiac dysfunction (shortness of breath at rest or exertion, orthopnea, exercise intolerance, lower-extremity edema, arrhythmias), were eligible for recruitment into the study. Other inclusion criteria included left ventricular ejection fraction (LVEF) ≥56%, R2 magnetic resonance imaging liver iron concentration (LIC) ≥3 mg Fe/g dry weight (dw), lifetime history of ≥50 U red blood cell transfusions, and receiving ≥10 U/y of red blood cell transfusions.
Patients with serum creatinine above the upper limit of normal (ULN) or significant proteinuria (urinary protein/creatinine ratio ≥1.0 mg/mg in a non–first-void urine sample at baseline) were excluded. In order to avoid excluding patients with increased alanine aminotransferase (ALT) levels due to liver iron overload, the ALT exclusion criterion was modified to exclude patients with ALT >5 times the ULN only if their LIC was <10 mg Fe/g dw. Other exclusion criteria included considerable impaired gastrointestinal (GI) function or GI disease, history of clinically relevant ocular and/or auditory toxicity related to iron chelation therapy, and history of HIV seropositivity or malignancy within the past 5 years.
Study design
CORDELIA was a prospective, multinational, randomized, open-label, parallel-group, phase 2 study conducted in 22 centers across 11 countries. Following a 35-day screening phase, patients were randomized in a 1:1 ratio to receive deferasirox (Exjade, Novartis) or DFO (Desferal, Novartis) for 1 year. Randomization was based on permuted blocks; stratification by center was not conducted. The once-daily deferasirox starting dose was 20 mg/kg per day for 2 weeks, followed by 30 mg/kg per day for 1 week, and then continued with 40 mg/kg per day. An intensified dosing regimen of DFO was administered, at 50 to 60 mg/kg per day via subcutaneous infusion over 8 to 12 hours, 5 to 7 days a week, in accordance with Thalassaemia International Federation guidelines.29 Dose adjustment recommendations were provided based on continuous assessment of efficacy and safety markers. Study medication was dispensed during regular study visits, and all medication returned by the patient was counted and recorded to assess compliance. Patients were instructed to contact the investigator if unable to take the study drug as prescribed.
The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki and was approved by institutional ethics committees at all participating sites. All patients or parents/guardians gave informed consent. An independent data-monitoring committee reviewed the safety data and advised on study continuation and/or any changes to protocol.
End points
Change in myocardial T2* was assessed as the ratio of the geometric mean (Gmean) T2* at end of study (EOS) divided by that at baseline (GmeanEOS/Gmeanbaseline). The primary efficacy end point was the ratio of Gmean myocardial T2* after 1 year of treatment with deferasirox divided by the ratio of Gmean for DFO.
A key secondary end point was to compare the 2 treatment groups for changes in LVEF after 1 year of treatment, assessed by absolute change from baseline CMR. Other end points included absolute change from baseline in LIC and serum ferritin after 1-year treatment with deferasirox and DFO.
Assessments
Efficacy was assessed using the per-protocol analysis set including all randomized patients treated for at least 6 months and with no major protocol violations. If the month 12 myocardial T2* value was not available, the last value obtained at ≥150 days was used. Patients without any T2* value after ≥150 days were excluded. Myocardial T2* and LVEF were measured with CMR at baseline, month 6, and EOS. A standardized CMR protocol for T2* acquisition technique2 was used and images were assessed by a central CMR core laboratory. LIC was measured using a validated R2 magnetic resonance imaging technique30 at baseline and then after 6 and 12 months of study treatment. Core laboratories were blinded to treatment allocation. Serum ferritin levels were assessed on blood samples drawn monthly from baseline to EOS. Monthly mean iron intake in mg/kg per day was determined based on the formula of average blood intake × 1.08/30 days.31
The safety set consisted of all randomized patients who received at least 1 dose of study drug and had at least 1 postbaseline safety assessment. Patients were analyzed according to treatment received (first study drug administered). Safety was evaluated through continuous monitoring and recording of adverse events (AEs), serious AEs (SAEs), laboratory testing, and clinical evaluations. Patients enrolled with a baseline myocardial T2* <10 milliseconds underwent additional 3-monthly monitoring for cardiac function and myocardial iron.
Myocardial iron concentration was derived from myocardial T2* values based on the formula described by Carpenter et al.1 Briefly, [Fe] = 45 × (T2*)−1.22, where [Fe] is measured in mg/g dw and T2* is measured in milliseconds. Ad hoc analysis of mean absolute change in myocardial iron concentration was thus conducted.
Statistical methods
Study sample size was determined for the primary end point of testing the noninferiority of deferasirox compared with DFO for ratio of T2* Gmeans with a 2-sided .05 α level, 85% power, and a noninferiority margin of 90%. Sample size accounted for an interim assessment using O’Brien–Fleming boundaries and for a potential 30% patient dropout before month 6. The analysis plan for the primary end point prespecified the per-protocol study population for testing of noninferiority, as per European Medicines Agency and International Conference on Harmonisation guidelines.32,33 A sensitivity analysis of the intention-to-treat population was planned. In order to eliminate potential unrecognized biases, the core clinical trial team was blinded to the treatment assignment prior to the database lock for the primary analysis.
For the primary efficacy variable, the 2-sided repeated 95% confidence interval (CI) for the ratio of T2* Gmeans of deferasirox over DFO was calculated. A noninferiority margin of 0.9 (90%) was applied because a loss of 10% efficacy relative to DFO was considered not clinically relevant. Noninferiority was therefore predefined if the lower limit of the repeated 95% CI for ratio of 2 Gmeans was >0.9. If noninferiority was established, a superiority test would be performed by comparing the lower limit of the repeated CI with 1. If the lower limit was >1, deferasirox would be declared superior to DFO; otherwise, deferasirox would not be declared superior. A 2-sided adjusted P value based on the Tsiatis, Rosner, and Mehta stage-wise ordering was calculated, testing superiority of deferasirox over DFO. Demographic and baseline characteristics and safety variables were summarized by frequency tables or summary statistics for continuous distributions.
Results
Patient disposition
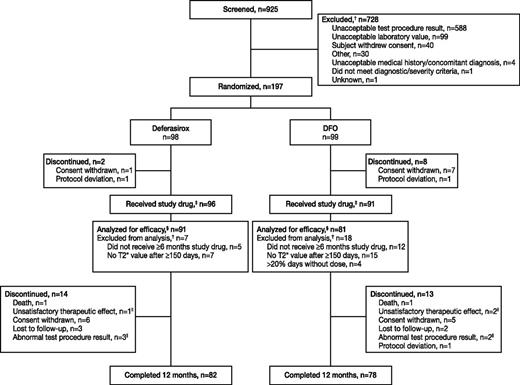
Overall, 925 patients were screened and 197 randomized (Figure 1). The majority of patients screened were β-thalassemia major patients (902/925; 99.1%). Other patients who were screened and for whom underlying anemia was captured had low/intermediate 1 myelodysplastic syndrome (n = 4), Diamond–Blackfan anemia, β-thalassemia intermedia, congenital dyserythropoietic anemia, and paroxysmal nocturnal hemoglobinuria (all n = 1). Only β-thalassemia major patients fulfilled the inclusion criteria and were enrolled in the study. A total of 81.2% of patients (n = 160) completed 1 year of treatment (Figure 1). Three patients in each group discontinued as a result of worsening of myocardial T2*.
Patient disposition. †A patient could have multiple reasons for screening failure or exclusion from efficacy analysis. ‡These patients comprised the safety set. §Efficacy was assessed using the per-protocol analysis set. ||Because of worsening of cardiac T2* in 3 deferasirox patients and 3 DFO patients, LVEF decreased <50% in 1 DFO patient and cardiomegaly in 1 deferasirox patient.
Patient disposition. †A patient could have multiple reasons for screening failure or exclusion from efficacy analysis. ‡These patients comprised the safety set. §Efficacy was assessed using the per-protocol analysis set. ||Because of worsening of cardiac T2* in 3 deferasirox patients and 3 DFO patients, LVEF decreased <50% in 1 DFO patient and cardiomegaly in 1 deferasirox patient.
Baseline demographics and clinical characteristics
Baseline demographics and clinical characteristics across deferasirox and DFO groups were similar (Table 1). The mean ± standard deviation (SD) age of patients was 19.8 ± 6.4 years. In patients randomized to deferasirox, Gmean (coefficient of variance) myocardial T2* at baseline was 11.2 (32.6) and 11.6 (30.7) in DFO patients. Patients were heavily iron overloaded, with baseline LIC of 30.0 ± 17.7 mg Fe/g dw and median (range) serum ferritin levels of 4878 (613-15 331) ng/mL.
Baseline patient characteristics
| Variable . | Deferasirox (n = 98) . | DFO (n = 99) . | All patients (n = 197) . |
|---|---|---|---|
| β-thalassemia major, n (%) | 98 (100) | 99 (100) | 197 (100) |
| Age, y | 19.9 ± 6.5 | 19.7 ± 6.3 | 19.8 ± 6.4 |
| Age range, y | 10.0-39.0 | 10.0-40.0 | 10.0-40.0 |
| Male:female, n | 58:40 | 57:42 | 115:82 |
| Race, n (%) | |||
| White | 59 (60.2) | 59 (59.6) | 118 (59.9) |
| Black | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Asian | 38 (38.8) | 40 (40.4) | 78 (39.6) |
| Other | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Hepatitis status, n (%) | |||
| Hepatitis B | 1 (1.0) | 2 (2.0) | 3 (1.5) |
| Hepatitis C | 22 (22.4) | 19 (19.2) | 41 (20.8) |
| Hepatitis B and C | 0 (0.0) | 2 (2.0) | 2 (1.0) |
| No hepatitis | 75 (76.5) | 80 (80.8) | 155 (78.7) |
| Time since start of blood transfusions, y | 19.3 ± 6.4 | 18.4 ± 6.2 | 18.8 ± 6.3 |
| Total number of blood transfusions received | 315.6 | 294.8 | 305.3 |
| Previous chelation therapy, n (%) | 96 (100) | 91 (100) | 187 (100) |
| DFO | 41 (42.7) | 39 (42.9) | 80 (42.8) |
| Deferiprone | 9 (9.4) | 5 (5.5) | 14 (7.5) |
| DFO + deferiprone | 21 (21.9) | 21 (23.1) | 42 (22.5) |
| Deferasirox | 18 (18.8) | 23 (25.3) | 41 (21.9) |
| Other† | 7 (7.3) | 3 (3.3) | 10 (5.3) |
| Time since start of first chelation therapy, y | 14.0 ± 7.0 | 14.3 ± 7.2 | 14.2 ± 7.1 |
| Myocardial T2* categories, n (%) | |||
| 6 to <10 ms | 33 (33.7) | 32 (32.3) | 65 (33.0) |
| 10 to ≤20 ms | 65 (66.3) | 67 (67.7) | 132 (67.0) |
| LVEF, n (%)‡ | |||
| <LLN by Westwood§ | 11 (12.1) | 10 (12.3) | 21 (12.2) |
| ≥LLN by Westwood§ | 80 (87.9) | 71 (87.7) | 151 (87.8) |
| LIC, mg Fe/g dw‡ | 29.8 ± 17.5 | 30.3 ± 17.9 | 30.0 ± 17.7 |
| LIC categories, n (%)‡ | |||
| LIC < 7 mg Fe/g dw | 11 (12.1) | 8 (9.9) | 19 (11.0) |
| LIC 7 to <15 mg Fe/g dw | 14 (15.4) | 14 (17.3) | 28 (16.3) |
| LIC ≥ 15 mg Fe/g dw | 66 (72.5) | 59 (72.8) | 125 (72.7) |
| Median serum ferritin (range), ng/mL‡ | 5062 (613-15 331) | 4684 (677-13 342) | 4878 (613-15 331) |
| Variable . | Deferasirox (n = 98) . | DFO (n = 99) . | All patients (n = 197) . |
|---|---|---|---|
| β-thalassemia major, n (%) | 98 (100) | 99 (100) | 197 (100) |
| Age, y | 19.9 ± 6.5 | 19.7 ± 6.3 | 19.8 ± 6.4 |
| Age range, y | 10.0-39.0 | 10.0-40.0 | 10.0-40.0 |
| Male:female, n | 58:40 | 57:42 | 115:82 |
| Race, n (%) | |||
| White | 59 (60.2) | 59 (59.6) | 118 (59.9) |
| Black | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Asian | 38 (38.8) | 40 (40.4) | 78 (39.6) |
| Other | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Hepatitis status, n (%) | |||
| Hepatitis B | 1 (1.0) | 2 (2.0) | 3 (1.5) |
| Hepatitis C | 22 (22.4) | 19 (19.2) | 41 (20.8) |
| Hepatitis B and C | 0 (0.0) | 2 (2.0) | 2 (1.0) |
| No hepatitis | 75 (76.5) | 80 (80.8) | 155 (78.7) |
| Time since start of blood transfusions, y | 19.3 ± 6.4 | 18.4 ± 6.2 | 18.8 ± 6.3 |
| Total number of blood transfusions received | 315.6 | 294.8 | 305.3 |
| Previous chelation therapy, n (%) | 96 (100) | 91 (100) | 187 (100) |
| DFO | 41 (42.7) | 39 (42.9) | 80 (42.8) |
| Deferiprone | 9 (9.4) | 5 (5.5) | 14 (7.5) |
| DFO + deferiprone | 21 (21.9) | 21 (23.1) | 42 (22.5) |
| Deferasirox | 18 (18.8) | 23 (25.3) | 41 (21.9) |
| Other† | 7 (7.3) | 3 (3.3) | 10 (5.3) |
| Time since start of first chelation therapy, y | 14.0 ± 7.0 | 14.3 ± 7.2 | 14.2 ± 7.1 |
| Myocardial T2* categories, n (%) | |||
| 6 to <10 ms | 33 (33.7) | 32 (32.3) | 65 (33.0) |
| 10 to ≤20 ms | 65 (66.3) | 67 (67.7) | 132 (67.0) |
| LVEF, n (%)‡ | |||
| <LLN by Westwood§ | 11 (12.1) | 10 (12.3) | 21 (12.2) |
| ≥LLN by Westwood§ | 80 (87.9) | 71 (87.7) | 151 (87.8) |
| LIC, mg Fe/g dw‡ | 29.8 ± 17.5 | 30.3 ± 17.9 | 30.0 ± 17.7 |
| LIC categories, n (%)‡ | |||
| LIC < 7 mg Fe/g dw | 11 (12.1) | 8 (9.9) | 19 (11.0) |
| LIC 7 to <15 mg Fe/g dw | 14 (15.4) | 14 (17.3) | 28 (16.3) |
| LIC ≥ 15 mg Fe/g dw | 66 (72.5) | 59 (72.8) | 125 (72.7) |
| Median serum ferritin (range), ng/mL‡ | 5062 (613-15 331) | 4684 (677-13 342) | 4878 (613-15 331) |
Values are mean ± SD unless otherwise stated.
LLN, lower limit of normal.
Unknown, or patients received irregular deferiprone and/or DFO therapy.
Based on the per-protocol population.
Westwood criteria: LLN, 59 for males and 63 for females.34
Exposure to treatment and compliance
Mean actual dose over 1-year treatment was 36.7 ± 4.2 mg/kg per day deferasirox (range, 19.7-43.3 mg/kg per day). Mean actual dose of DFO was 41.5 ± 8.7 (13.2-60.2) mg/kg per day, when normalized to a 7-day regimen. The maximum dose used at any time during the study was 49.9 mg/kg per day deferasirox and 62.5 mg/kg DFO. As a result of rounding to the nearest whole-tablet strength, 8 patients received deferasirox doses >40 mg/kg per day.
Patients received study drug for a median duration of 355.5 days (range, 3.0-418.0) and 355.0 days (range, 86.0-394.0) in the deferasirox and DFO cohort, respectively. Total exposure was 87.8 patient-years for deferasirox patients and 81.5 patient-years for DFO patients. Overall, deferasirox patients took 99.0% ± 3.5% of the planned dose and DFO patients took 100.4% ± 10.9%. Dose was interrupted at least once in 18.8% of deferasirox patients and in 17.6% of DFO patients. The main reason for interruption was an AE (n = 21 [21.9%] vs n = 19 [20.9%], respectively). Dose was reduced at least once in 15.6% and 19.8% of patients, respectively; the main reason was also an AE (n = 24 [25.0%] vs n = 21 [23.1%]).
Average iron intake
Average iron intake throughout the study was <0.3 mg/kg per day in 55.2% of patients (deferasirox, 53.8%; DFO, 56.8%), 0.3 to 0.5 mg/kg per day in 36.6% of patients (deferasirox, 38.5%; DFO, 34.6%), and >0.5 mg/kg per day in 8.1% of patients (deferasirox, 7.7%; DFO, 8.6%) and was similar between groups.
Efficacy of deferasirox compared with DFO
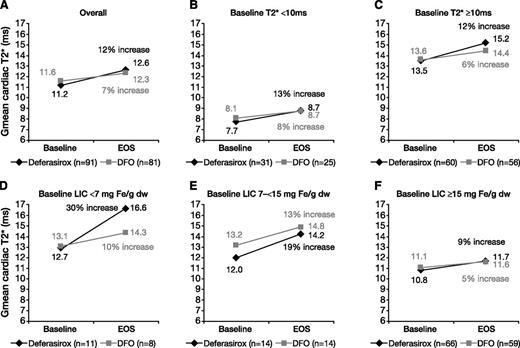
Myocardial T2* improved after 1 year of treatment (Table 2). In the per-protocol population, Gmean (coefficient of variance) myocardial T2* improved after 1 year of treatment with deferasirox by 12% (11.2 [32.6] milliseconds at baseline to 12.6 [42.6] milliseconds at EOS) and by 7% for DFO (11.6 [30.7] milliseconds to 12.3 [34.7] milliseconds; Figure 2A). The ratio of the Gmeans of deferasirox over DFO was 1.056 (repeated 95% CI, 0.998, 1.133). Because the lower bound of the 95% CI was greater than prespecified margin of 0.9, noninferiority of deferasirox compared with DFO for myocardial iron removal was demonstrated. A trend for superiority of deferasirox compared with DFO was observed, although this did not reach statistical significance (P = .057). An analysis of the intention-to-treat population showed similar results to the per-protocol population (Table 2).
Comparison of myocardial T2* change from baseline in patients treated with deferasirox or DFO for 1 year
| . | Deferasirox . | DFO . | All patients . |
|---|---|---|---|
| Per-protocol population | n = 91 | n = 81 | n = 172 |
| Gmean T2* (CV) at baseline | 11.2 (32.6) | 11.6 (30.7) | 11.4 (31.7) |
| Gmean T2* (CV) at EOS† | 12.6 (42.6) | 12.3 (34.7) | 12.5 (38.9) |
| Gmean ratio of month 12/baseline (95% CI) | 1.12 (1.07, 1.18) | 1.07 (1.02, 1.11) | 1.10 (1.06, 1.13) |
| Ratio of Gmean ratios of deferasirox vs DFO | — | — | 1.056 |
| Repeated 95% CI of the ratio of Gmean ratios | — | — | (0.998, 1.133) |
| P value for superiority‡ | — | — | .057 |
| Intention-to-treat population | n = 92 | n = 88 | n = 180 |
| Gmean T2* (CV) at baseline | 11.2 (31.9) | 11.6 (32.9) | 11.4 (32.4) |
| Gmean T2* (CV) at EOS† | 12.5 (43.0) | 12.0 (36.3) | 12.2 (39.7) |
| Gmean ratio of month 12/baseline (95% CI) | 1.12 (1.07, 1.18) | 1.06 (1.02, 1.11) | 1.09 (1.06, 1.13) |
| Ratio of Gmean ratios of deferasirox vs DFO | — | — | 1.055 |
| Repeated 95% CI of the ratio of Gmean ratios | — | — | (0.999, 1.129) |
| P value for superiority‡ | — | — | .054 |
| . | Deferasirox . | DFO . | All patients . |
|---|---|---|---|
| Per-protocol population | n = 91 | n = 81 | n = 172 |
| Gmean T2* (CV) at baseline | 11.2 (32.6) | 11.6 (30.7) | 11.4 (31.7) |
| Gmean T2* (CV) at EOS† | 12.6 (42.6) | 12.3 (34.7) | 12.5 (38.9) |
| Gmean ratio of month 12/baseline (95% CI) | 1.12 (1.07, 1.18) | 1.07 (1.02, 1.11) | 1.10 (1.06, 1.13) |
| Ratio of Gmean ratios of deferasirox vs DFO | — | — | 1.056 |
| Repeated 95% CI of the ratio of Gmean ratios | — | — | (0.998, 1.133) |
| P value for superiority‡ | — | — | .057 |
| Intention-to-treat population | n = 92 | n = 88 | n = 180 |
| Gmean T2* (CV) at baseline | 11.2 (31.9) | 11.6 (32.9) | 11.4 (32.4) |
| Gmean T2* (CV) at EOS† | 12.5 (43.0) | 12.0 (36.3) | 12.2 (39.7) |
| Gmean ratio of month 12/baseline (95% CI) | 1.12 (1.07, 1.18) | 1.06 (1.02, 1.11) | 1.09 (1.06, 1.13) |
| Ratio of Gmean ratios of deferasirox vs DFO | — | — | 1.055 |
| Repeated 95% CI of the ratio of Gmean ratios | — | — | (0.999, 1.129) |
| P value for superiority‡ | — | — | .054 |
CV, coefficient of variance.
Last available value at least 150 days after randomization.
Two-sided adjusted P value based on the Tsiatis, Rosner, and Mehta stage-wise ordering.
Gmean myocardial T2* in patients treated with deferasirox or DFO for 1 year. Values are for (A) all patients or patients with (B) baseline myocardial T2* <10 milliseconds, (C) baseline myocardial T2* ≥10 milliseconds, (D) baseline LIC <7 mg Fe/g dw, (E) baseline LIC 7 to <15 mg Fe/g dw, or (F) baseline LIC ≥15 mg Fe/g dw. All panels are based on the per-protocol population.
Gmean myocardial T2* in patients treated with deferasirox or DFO for 1 year. Values are for (A) all patients or patients with (B) baseline myocardial T2* <10 milliseconds, (C) baseline myocardial T2* ≥10 milliseconds, (D) baseline LIC <7 mg Fe/g dw, (E) baseline LIC 7 to <15 mg Fe/g dw, or (F) baseline LIC ≥15 mg Fe/g dw. All panels are based on the per-protocol population.
Myocardial T2* improved with deferasirox and DFO treatment in patients who had T2* below or above 10 milliseconds at baseline (Figure 2B-C). In patients with baseline LIC <7 mg Fe/g dw, increase from baseline in myocardial T2* was 30% (n = 11) for deferasirox and 10% for DFO (n = 8), and for patients with baseline LIC ≥15 mg Fe/g dw, increase was 9% (n = 66) and 5% (n = 59), respectively (Figure 2D-F).
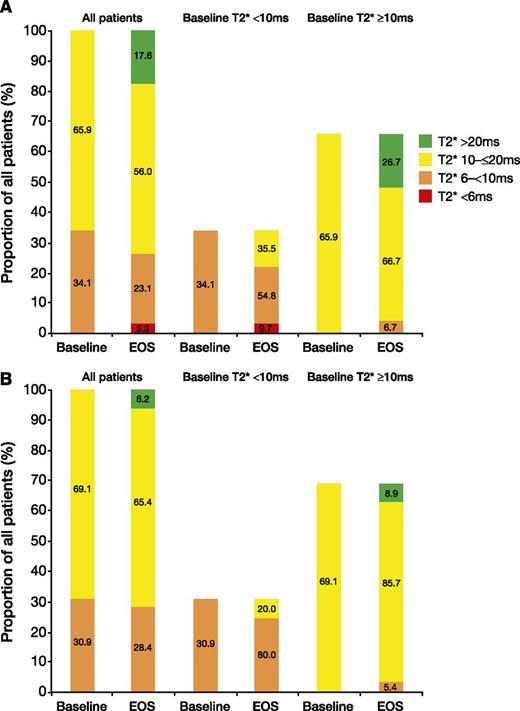
After 1 year, 16 (17.6%) patients treated with deferasirox normalized their myocardial T2*, and 11 (35.5%) patients improved from a baseline myocardial T2* of 6 to <10 milliseconds to 10 to ≤20 milliseconds at EOS (Figure 3A). In comparison, 5 (6.2%) DFO patients overall normalized their myocardial T2*, and 5 (20.0%) patients treated with DFO improved from a baseline myocardial T2* of 6 to <10 milliseconds to 10 to ≤20 milliseconds at EOS (Figure 3B). Overall, 4 (6.7%) and 3 (5.4%) patients treated with deferasirox and DFO worsened to 6 to <10 milliseconds from a baseline of 10 to ≤20 milliseconds. These results are based on the per-protocol analysis population and do not take into account patients discontinuing as a result of worsening of cardiac T2* prior to month 6.
Shift in proportion of patients with severe, mild-to-moderate, and normalized cardiac T2* values at baseline and EOS. Values are for patients treated with (A) deferasirox or (B) DFO for 1 year. Both panels are based on the per-protocol population.
Shift in proportion of patients with severe, mild-to-moderate, and normalized cardiac T2* values at baseline and EOS. Values are for patients treated with (A) deferasirox or (B) DFO for 1 year. Both panels are based on the per-protocol population.
Effect of deferasirox compared with DFO on cardiac function
Mean LVEF remained stable and within the normal range after 1 year of treatment with deferasirox (66.9% ± 5.6% at baseline to 66.3% ± 5.8% at EOS) and DFO (66.4% ± 5.2% to 66.4% ± 5.8%). Change in mean LVEF after 1 year was not different between the 2 treatments (P = .54). Of patients with abnormal LVEF34 at baseline, 6 (54.5%) deferasirox patients and 5 (50.0%) DFO patients had improved LVEF to within the normal range. Overall, 7 (8.8%) deferasirox patients and 9 (12.7%) DFO patients who had LVEF in the normal range at baseline had decreased LVEF to below LLN by EOS.34
Other iron parameters
Myocardial iron concentration.
After 1 year of treatment with deferasirox, myocardial iron concentration decreased from a baseline of 2.6 ± 1.0 mg Fe/g dw to 2.3 ± 1.2 mg Fe/g dw (absolute change from baseline, −0.24 ± 0.7 mg Fe/g dw; 95% CI, −0.1, −0.4). In patients treated with DFO, myocardial iron decreased from 2.4 ± 0.9 mg Fe/g dw at baseline to 2.3 ± 0.9 mg Fe/g dw at EOS (absolute change from baseline, −0.15 ± 0.5 mg Fe/g dw; 95% CI, −0.03, 0.3). Decreases in myocardial iron were observed in all subgroups examined (supplemental Table 1, available on the Blood Web site).
LIC.
After 1 year of treatment with deferasirox, LIC decreased from 29.8 ± 17.5 mg Fe/g dw at baseline to 20.1 ± 17.5 mg Fe/g dw at EOS (absolute change from baseline, −8.9 ± 11.4 mg Fe/g dw; 95% CI, −11.5, −6.4). LIC decreased from a baseline of 30.3 ± 17.9 to 17.7 ± 14.4 mg Fe/g dw in DFO patients (change from baseline, −12.7 ± 11.4 mg Fe/g dw; 95% CI, −15.3, −10.1).
Serum ferritin level.
Treatment with deferasirox for 1 year reduced serum ferritin levels from a baseline of 5062 (613-15 331) ng/mL to 3375 (346-31 942) ng/mL at EOS (absolute change from baseline, −1044 [−5561-18 838] ng/mL). In DFO patients, serum ferritin levels reduced from 4684 (677-13 342) ng/mL at baseline to 3129 (470-9487) ng/mL after 1 year (change from baseline, −1277 [−7577-2810] ng/mL).
Safety parameters
AEs.
Investigator-reported AEs, regardless of causality, were reported in 65 (67.7%) deferasirox patients and 69 (75.8%) DFO patients (supplemental Table 2). AEs suspected to be related to study drug occurred in 35.4% of deferasirox patients and 30.8% of DFO patients; the most common (≥5%) were increased blood creatinine (8.3% vs 2.2%, respectively), proteinuria (7.3% vs 3.3%), increased ALT (6.3% vs 1.1%), increased aspartate aminotransferase (6.3% vs 1.1%), and diarrhea (6.3% vs 1.1%) (supplemental Table 3).
SAEs, irrespective of causality, were reported in 10 (10.4%) deferasirox patients and 10 (11.0%) DFO patients (supplemental Table 2). Of these, 2 SAEs in 1 patient (vomiting and upper abdominal pain) were suspected to be related to deferasirox and 2 SAEs (GI infection and meningitis) were suspected to be related to DFO.
One deferasirox patient experienced an AE (arrhythmia) leading to study drug discontinuation, which was not suspected to be related to study drug. Three DFO patients had AEs that led to study drug discontinuation: meningitis and neurosensory deafness suspected to be related to treatment and myocardial T2* <6 milliseconds not suspected related to DFO. Two deaths occurred during the study, both following AEs leading to discontinuation. One death in the deferasirox arm was due to arrhythmia and was not suspected to be related to study drug. The other death in a DFO patient was due to meningitis and was suspected by the investigator to be related to study drug, with splenectomy and progression of diabetes considered possible contributory factors.
Laboratory parameters.
Overall, 3 (3.1%) patients in the deferasirox cohort and 1 (1.1%) in the DFO cohort had 2 consecutive serum creatinine increases of >33% above baseline values and above the ULN. Increases were transient and managed with dose reduction and/or interruption. Increased blood creatinine was also reported as an AE in all 4 patients. After 1 year of treatment, mean ± SD creatinine clearance had decreased in both deferasirox (−37.0 ± 42.9 mL/min) and DFO patients (−23.1 ± 36.6 mL/min), although on average no progressive decreases were observed.
Mean ± SD baseline ALT was elevated in both deferasirox (71.6 ± 84.0 units/liter [U/L]) and DFO (58.7 ± 44.5 U/L) treatment arms. Among patients with abnormal baseline ALT, levels had improved to within the normal range in 20 (31.7%) and 22 (39.3%) patients after 1 year of treatment with deferasirox or DFO, respectively. Overall, mean ALT levels decreased during treatment with deferasirox (month 12: 54.2 ± 83.9 U/L; change from baseline, −3.5 ± 80.4 U/L) and with DFO (46.3 ± 42.2 U/L; 18.9 ± 35.5 U/L). During the study, 6 (3.2%) patients had 2 consecutive ALT increases >5× ULN and 2× baseline, including 4 (4.2%) deferasirox patients and 2 (2.2%) DFO patients. ALT increases in deferasirox patients were transient and resolved with dose interruption (in 2 patients) or without intervention (in 1 patient). In the remaining deferasirox patient, this was noted at the last visit on record and no follow-up information was available. In DFO patients, ALT levels returned to baseline after dose interruption in 1 patient and without intervention in the second patient.
Discussion
Myocardial siderosis remains a common cause of death in patients with β-thalassemia major, and there is therefore a need to optimize chelation regimens specifically for myocardial iron removal.6-9 CORDELIA was the first randomized controlled trial comparing deferasirox with an intensified DFO regimen for myocardial iron removal in patients with β-thalassemia major. The study met its primary end point in demonstrating noninferiority of deferasirox compared with DFO. After 1 year of treatment, myocardial T2* improved by 12% from baseline with deferasirox and by 7% in patients treated with DFO. There was a trend toward superiority for deferasirox, which failed to meet conventional significance (P = .057). The importance of deferasirox as a noninferior alternative treatment of cardiac siderosis to DFO lies in its oral preparation, which is preferable to injected DFO, which may have substantial long-term compliance problems.
There are few randomized controlled trials assessing the efficacy and safety of iron chelation therapy in β-thalassemia major patients with myocardial iron overload.14,22,25 Although CORDELIA adds to this body of data, additional well-designed randomized comparisons would still be valuable. A comparison of deferiprone and DFO in 61 patients by Pennell et al showed that improvement in myocardial T2* was significantly greater for deferiprone than DFO (27% vs 13%; P = .023) over 1 year.14 Patients treated with deferiprone (n = 29) had baseline myocardial T2* of 13.0 milliseconds, and patients treated with DFO (n = 32) had T2* of 13.3 milliseconds. A study by Tanner et al reports that in 65 patients, myocardial T2* improved by 50% in patients receiving deferiprone and DFO combination therapy and by 24% in patients receiving DFO alone.22 In all treatment arms, including DFO, improvements in myocardial T2* in these 2 studies were greater than those observed in CORDELIA. However, differences observed between the CORDELIA study and prior studies also need to be interpreted in the light of baseline patient demographics in the respective studies and differences in treatment doses of DFO. It is notable, for example, that baseline LIC in both the Pennell et al and Tanner et al studies was significantly lower than in the CORDELIA study, which may impact cardiac T2* response. In the Pennell et al study, for example, patients randomized to deferiprone and DFO had LIC reported as 6.2 ± 6.0 mg Fe/g dw and 6.3 ± 5.8 mg Fe/g dw, respectively.14 Even when taking into account underestimation (around 50%)35 by superconducting quantum interference device measurements used in this previous study, the baseline LIC levels observed in CORDELIA patients (30.0 ± 17.7 mg Fe/g dw) remain higher. This is also confirmed by differences in serum ferritin levels (mean of 1791 ng/mL in deferiprone patients and 2795 ng/mL in DFO patients14 vs a median of 4878 ng/mL in CORDELIA patients). Serum ferritin levels were also lower in the Tanner et al study (1574 and 1379 ng/mL for combined deferiprone and DFO vs DFO alone, respectively)22 compared with CORDELIA. Wood et al have reported that baseline LIC and serum ferritin levels are clinically relevant predictors of cardiac response to deferasirox therapy.23 This is consistent with results from CORDELIA, in which patients with LIC <7 mg Fe/g dw treated with deferasirox showed a trend toward greater improvement in myocardial T2* compared with patients with higher baseline LIC.
Further differences between the CORDELIA study and other randomized comparisons included patient demographics, study design, and dose of DFO. Pennell et al reported a DFO dose equivalent to 35 mg/kg per day for 7 days per week14 and Tanner et al reported 31.0 mg/kg per day for 7 days per week,22 whereas the dose in CORDELIA was higher at 41.5 mg/kg per day for 7 days per week. Lower DFO dosing might have favored deferiprone (or deferiprone plus DFO combination). Although patient compliance can be a concern with DFO treatment, patient-reported adherence to study drug regimen was very good in CORDELIA. DFO compliance as assessed by the percentage of completed infusions in the 2 studies by Pennell et al and the Tanner et al was also >90%.14,22
Longitudinal studies of up to 3 years of treatment with deferasirox have shown improvements in myocardial T2* over time.20,23,26 Because heart iron clearance is slower than that of the liver,10 normalization of myocardial T2* may take several years. To that end, a 1-year extension for CORDELIA is currently ongoing. Nevertheless, over a period of 1 year, we observed normalization of myocardial T2* in 17.6% of deferasirox patients compared with 6.2% of DFO patients overall. Importantly, 35.5% of deferasirox patients and 20.0% of DFO patients with severe myocardial siderosis improved to the mild-to-moderate category after 1 year of treatment. Three patients in each treatment group discontinued the study as a result of worsening of myocardial T2*.
Improvements in LVEF have been shown after treatment with either deferiprone monotherapy or in combination with DFO.14,22 In CORDELIA, LVEF remained stable during the study period. Other prospective clinical trials with deferasirox in patients with myocardial siderosis have also shown no change in LVEF for treatment periods up to 3 years.20,23,26,28 In contrast, recent results from a small study of 13 patients with T2* from 10 to 20 milliseconds treated with deferasirox for 32 ± 7 months showed improvement in LVEF, albeit from lower baseline values of 59.8%.36 In CORDELIA, approximately half of both the deferasirox and DFO patients who had low LVEF at baseline improved to within the normal range34 by EOS. This may be important, because even small improvements in LVEF can reduce the risk of heart failure, even in β-thalassemia patients with LVEF in the normal range.37 However, 8.8% of deferasirox patients and 12.7% of DFO patients decreased LVEF to below LLN.
Rates of dose reduction and/or interruption were similar between treatment groups, and frequency of AEs was also similar. The safety profile of deferasirox was comparable to previous reports, with the most common drug-related AEs being increased laboratory parameters and diarrhea.20,23,26 Two deaths occurred during the study. The death of the patient treated with deferasirox was due to arrhythmia and was not considered related to study drug. The death of the DFO patient who died as a result of meningitis was suspected by the investigator to be related to DFO, with splenectomy and progression of diabetes identified as possible contributory factors.
In conclusion, the randomized controlled trial CORDELIA met its primary end point, demonstrating noninferiority of once-daily oral treatment with deferasirox compared with DFO for the removal of myocardial iron, with a trend toward superiority for deferasirox. These data add to the body of knowledge allowing physicians to make best-informed choices for their patients.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Debbi Gorman of Mudskipper Business Ltd for medical editorial assistance. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals.
This study was sponsored by Novartis Pharma AG and designed by the sponsor in close collaboration with the Study Steering Committee. The sponsor conducted the statistical analysis. Authors had full access to the data and participated actively in interpreting data and writing and critically reviewing the article with the assistance of a medical writer funded by the sponsor. The corresponding author had final responsibility for the manuscript content and decision to submit for publication.
Authorship
Contribution: J.B.P., Y.L., A.E.-B., K.M.B., M.E., A.Y., Y.K., and Y.A. served as investigators on this trial, enrolling patients, contributing to data interpretation, and reviewing and providing comments on this manuscript; D.J.P., J.B.P., A.P., and Y.A. served as Study Steering Committee members overseeing the conduct of the trial, from study design to analysis plan and data interpretation; T.L., D.H., and M.W. assisted in developing the trial protocol, coordinating the execution of the trial, and contributing to the analysis, interpretation, and reporting of the trial data; Y.Z. served as the trial statistician; and all authors approved the final manuscript.
Conflict-of-interest disclosure: D.J.P. is a consultant for and receives research grant funding and honoraria from Novartis Pharmaceuticals and AMAG Pharmaceuticals, receives lecture fees from Novartis Pharmaceuticals, is a consultant for and receives honoraria from ApoPharma and Shire, and is a director and equity holder in Cardiovascular Imaging Solutions. Y.A. participates in advisory boards and speaker’s bureaus and receives honoraria and research grant funding from Novartis Pharmaceuticals and participates in advisory boards and receives research grant funding from Shire. J.B.P. is a consultant for and receives research grant funding and honoraria from Novartis Pharmaceuticals, is a consultant for and receives research grant funding from Shire, and is a consultant for Celgene. A.P. participates in advisory boards and receives research grant funding from Novartis Pharmaceuticals. D.H. and Y.Z. are employees of Novartis Pharmaceuticals, and T.L. and M.W. are employees of Novartis Pharma AG. The remaining authors declare no competing financial interests.
Correspondence: Dudley J. Pennell, National Institute for Health Research Cardiovascular Biomedical Research Unit, Royal Brompton Hospital, Sydney Street, London, SW3 6NP, United Kingdom; e-mail: d.pennell@ic.ac.uk.