Key Points
Leukocyte cell-derived chemotaxin-2–associated amyloidosis (ALect2) is a common cause of systemic amyloidosis involving the liver.
Recognition and accurate diagnosis of hepatic ALect2 amyloidosis is essential for accurate management of patients with hepatic amyloidosis.
Abstract
Using laser microdissection and mass spectrometry (MS)-based proteomics, we subtyped amyloid deposits from 130 cases of hepatic amyloidosis. Although we confirmed that immunoglobulin light chain amyloidosis was the most frequent cause of hepatic amyloidosis, leukocyte cell-derived chemotaxin 2 (LECT2) amyloidosis (ALect2) accounted for 25% of cases. This novel finding was associated with Hispanic ancestry, incidental discovery of amyloid in liver specimens sampled for other unrelated conditions, and a characteristic pattern of hepatic amyloid deposition. Although ALect2 patients had a common LECT2 polymorphism, pathogenic mutations were not discovered, suggesting that constitutive or compensatory LECT2 overexpression led to ALect2 deposition. These findings indicate that ALect2 is a common cause of hepatic amyloidosis in the population of the United States, and subtyping hepatic amyloid deposits by an accurate analytic method such as MS is required for optimal clinical management of hepatic amyloidosis patients and to avoid incorrect and unnecessarily toxic therapies.
Introduction
The liver is a frequent site of involvement by systemic amyloidosis where 60% to 90% of cases have been reported to show liver involvement at autopsy.1 While immunoglobulin light chain amyloidosis (AL) and serum amyloid A protein (AA) are reported to be the most frequent types of hepatic amyloid, these subtypes were often assumed based on clinical context or suboptimal techniques rather than directly demonstrated by an untargeted objective analytical method. We recently established an accurate clinical assay for assigning amyloid types using laser microdissection (LMD) and proteomic analysis by liquid chromatography/tandem mass spectrometry (MS).2-8 The aim of this study was to gain further insight into the nature of hepatic amyloid deposits using LMD/MS to support optimal management of hepatic amyloidosis patients. Here we show that although AL is the most frequent type of hepatic amyloid, leukocyte cell-derived chemotaxin 2 (LECT2)-associated amyloidosis (ALect2) accounts for a quarter of the cases. Recognition of ALect2 as a major cause of hepatic amyloidosis requires reassessment of the management of these patients.
Study design
We prospectively studied 130 consecutive cases of amyloidosis involving the liver submitted for LMD/MS analysis between 2009 and 2012. Detailed clinical data were available in 86 of the cases. This study was approved by the Mayo Clinic Institutional Review Board and was conducted in accordance with the Declaration of Helsinki.
For each case, hematoxylin and eosin and Congo-red stained sections were examined to confirm the presence of amyloid and to assess the distribution (perisinusoidal or portal) and pattern (nodular, globular, or vascular) of hepatic involvement. The amyloid type was identified by LMD/MS as previously described.2 LECT2 protein and messenger RNA (mRNA) were detected by immunohistochemistry (IHC) and in situ hybridization (ISH), respectively, on liver sections of Alect2 amyloidosis, and on microarrays representing normal, inflammatory, and neoplastic tissues. LECT2 mutation analysis was performed in a case of hepatic ALect2 amyloidosis and in 9 additional cases of renal ALect2 amyloidosis (see the supplemental Methods on the Blood Web site).
Results and discussion
Clinical classification of hepatic amyloidosis by LMD/MS-based proteomic assay
LMD/MS identified the causative protein in every case. AL was the most common amyloidosis type, affecting 81 (62%) patients (AL-λ, 45 cases [35%]; AL-κ, 36 cases [28%]). ALect2 amyloidosis was the second most frequent type of hepatic amyloidosis (32 cases [25%]) (Figure 1A). ALect2 has been recently described as a novel type of renal amyloidosis.9-11 As described, slowly progressing chronic renal disease without systemic dissemination characterizes renal Lect2 amyloidosis. However, our findings show that ALect2 amyloidosis is also a frequent cause of hepatic, and therefore, systemic amyloidosis in the population of the United States. Nine AApoAI (7%), 5 AA (4%), 2 ATTR (2%), and 1 ALys (1%) constituted the remaining cases.
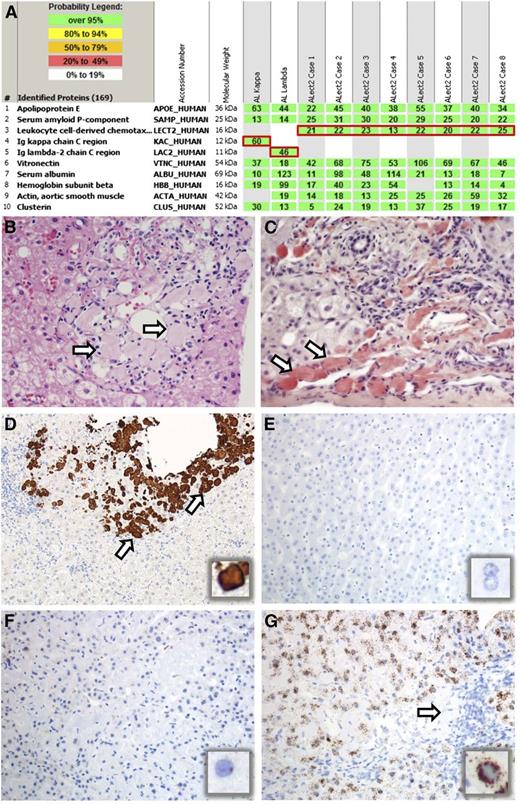
LMD/MS based proteomic findings in 10 cases of hepatic amyloidosis and pathological features of ALect2 amyloidosis. (A) LMD/MS read-outs of 2 cases of AL and 8 cases of ALect2 amyloidosis. The proteins identified are listed according to relative abundance based on spectral counts. The first five proteins represent amyloid-associated proteins identified in this cohort, followed by other abundant proteins identified in the deposits. The first two most abundant amyloid-associated proteins (1-2) across the cohort and present in every amyloid type are apolipoprotein E and serum amyloid P-component. The following three proteins (3-5) highlighted by red boxes represent pathogenic proteins identified in these 10 cases. Only one pathogenic protein is present in each case. Rows 1 and 2 represent AL, and rows 3-10 ALect2 amyloidosis. (B-C) Histologic features of ALect2 amyloidosis. There are periportal globular deposits of proteinaceous material (B, arrows) that are strongly Congo-red positive (C, arrows). (B: hematoxylin and eosin, C: Congo red, ×200 original magnification; image acquired using an Olympus DP71 camera and Olympus BX51 microscope.) (D-G) Expression of LECT2 in ALect2 amyloidosis (D-G), normal liver (E), and AL amyloidosis (F). IHC for LECT2 protein labels the globular amyloid deposits in ALect2 amyloidosis (D, arrows and inset), whereas the surrounding hepatic parenchyma is entirely negative. ISH for LECT2 mRNA is negative in normal liver (E, and inset), rare cells are weakly positive in AL amyloidosis (F, and inset), whereas most cells are strongly positive in ALect2 amyloidosis (G and inset; arrow indicates globular amyloid deposits). (D: IHC for LECT2, ×40 original magnification; E-F: ISH for LECT2 mRNA, ×100 original magnification. All images were acquired using an Olympus DP73 camera and Olympus BX51 microscope.)
LMD/MS based proteomic findings in 10 cases of hepatic amyloidosis and pathological features of ALect2 amyloidosis. (A) LMD/MS read-outs of 2 cases of AL and 8 cases of ALect2 amyloidosis. The proteins identified are listed according to relative abundance based on spectral counts. The first five proteins represent amyloid-associated proteins identified in this cohort, followed by other abundant proteins identified in the deposits. The first two most abundant amyloid-associated proteins (1-2) across the cohort and present in every amyloid type are apolipoprotein E and serum amyloid P-component. The following three proteins (3-5) highlighted by red boxes represent pathogenic proteins identified in these 10 cases. Only one pathogenic protein is present in each case. Rows 1 and 2 represent AL, and rows 3-10 ALect2 amyloidosis. (B-C) Histologic features of ALect2 amyloidosis. There are periportal globular deposits of proteinaceous material (B, arrows) that are strongly Congo-red positive (C, arrows). (B: hematoxylin and eosin, C: Congo red, ×200 original magnification; image acquired using an Olympus DP71 camera and Olympus BX51 microscope.) (D-G) Expression of LECT2 in ALect2 amyloidosis (D-G), normal liver (E), and AL amyloidosis (F). IHC for LECT2 protein labels the globular amyloid deposits in ALect2 amyloidosis (D, arrows and inset), whereas the surrounding hepatic parenchyma is entirely negative. ISH for LECT2 mRNA is negative in normal liver (E, and inset), rare cells are weakly positive in AL amyloidosis (F, and inset), whereas most cells are strongly positive in ALect2 amyloidosis (G and inset; arrow indicates globular amyloid deposits). (D: IHC for LECT2, ×40 original magnification; E-F: ISH for LECT2 mRNA, ×100 original magnification. All images were acquired using an Olympus DP73 camera and Olympus BX51 microscope.)
Clinical and pathological features of hepatic ALect2 amyloidosis
ALect2 amyloidosis produced a number of unique clinical and pathological features (Table 1). Similar to AL amyloidosis, the median patient age was 60.5 years (range = 33 to 79 years) with male/female ratio of 0.6:1. Like renal ALect2 amyloidosis,11,12 hepatic ALect2 patients were usually of Hispanic ethnicity (n = 28). Most patients with hepatic AL amyloid presented with liver abnormalities (most frequently hepatomegaly and elevated liver functions tests) attributed to the hepatic amyloidosis. By contrast, in ALect2 patients, the diagnosis of amyloidosis was often incidentally discovered during evaluations for conditions unrelated to the liver (odds ratio = 4.9737; P = .0185), or was associated with other causes of liver disease such as chronic viral hepatitis or steatohepatitis (Table 1 and supplemental Table 1). These observations suggest that hepatic ALect2 amyloidosis may not be a cause of clinically significant liver disease. Except for 1 patient with diabetes and chronic renal disease, none of the patients with hepatic ALect2 amyloidosis had evidence of kidney disease at the time of presentation.
Clinicopathological features of ALect2 amyloidosis
| Case . | Age (y) . | Sex . | Ethnicity . | Presentation . | Histological pattern . | Other pathology . | MS results . | LECT2 IHC . |
|---|---|---|---|---|---|---|---|---|
| 1 | 65 | M | Caucasian | N/A | Portal/globular | Steatohepatitis | ALect2 | + |
| 2 | 41 | M | Caucasian | N/A | Portal/globular | Chronic hepatitis C | ALect2 | + |
| 3 | 60 | M | Caucasian | Incidental* | Portal/globular | Chronic hepatitis C | ALect2 | + |
| 4 | 70 | F | Hispanic | Cirrhosis, ascites, hepatitis | Portal/globular | Chronic hepatitis unknown type | ALect2 | + |
| 5 | 52 | F | Hispanic | Elevated LFTs | Portal/globular | Steatohepatitis | ALect2 | + |
| 6 | 65 | M | Hispanic | Elevated LFTs | Portal/globular | - | ALect2 | + |
| 7 | 76 | M | Hispanic | N/A | Portal/globular | - | ALect2 | + |
| 8 | 65 | F | Hispanic | Incidental* | Portal/globular | Steatosis | ALect2 | + |
| 9 | 62 | F | Hispanic | Incidental* | Portal/globular | Lymphocytic infiltrate | ALect2 | + |
| 10 | 58 | F | Caucasian | Incidental* | Portal/nodular | - | ALect2 | + |
| 11 | 62 | F | Hispanic | Elevated LFTs | Portal/globular | - | ALect2 | + |
| 12 | 36 | M | Hispanic | Elevated LFTs | Portal/globular | Lymphocytic infiltrate | ALect2 | + |
| 13 | 72 | F | Hispanic | Incidental* | Portal/nodular | - | ALect2 | + |
| 14 | 60 | F | Hispanic | N/A | Portal/globular | Lymphocytes infiltrate | ALect2 | + |
| 15 | 61 | F | Hispanic | Elevated LFTs | Portal/globular | Steatosis | ALect2 | + |
| 16 | 52 | F | Hispanic | Incidental* | Portal/globular | Cirrhosis, steatohepatitis | ALect2 | + |
| 17 | 79 | F | Hispanic | Elevated LFTs | Portal/globular | - | ALect2 | + |
| 18 | 63 | M | Hispanic | Elevated LFTs | Portal/globular | Cirrhosis | ALect2 | + |
| 19 | 54 | F | Hispanic | Elevated LFTs | Portal/globular | Steatosis, lymphocytic infiltrate | ALect2 | + |
| 20 | 74 | F | Hispanic | Incidental* | Portal/globular | Cirrhosis | ALect2 | + |
| 21 | 59 | M | Hispanic | N/A | Portal/globular | - | ALect2 | + |
| 22 | 61 | M | Hispanic | N/A | Portal/globular | - | ALect2 | + |
| 23 | 69 | M | Hispanic | N/A | Portal/globular | Chronic hepatitis C, portal fibrosis | ALect2 | + |
| 24 | 59 | M | Hispanic | N/A | Portal/globular | Lymphocytic infiltrate | ALect2 | + |
| 25 | 52 | F | Hispanic | N/A | Portal/globular | - | ALect2 | + |
| 26 | 53 | F | Hispanic | N/A | Portal/globular | - | ALect2 | + |
| 27 | 79 | F | Hispanic | N/A | Portal/globular | - | ALect2 | + |
| 28 | 66 | F | Hispanic | N/A | Portal/globular | Lymphocytic infiltrate | ALect2 | + |
| 29 | 33 | F | Hispanic | N/A | Portal/globular | Steatosis | ALect2 | + |
| 30 | 52 | M | Hispanic | Portal hypertension | Portal/globular | Steatohepatitis | ALect2 | + |
| 31 | 59 | F | Hispanic | Elevated LFTs | Portal/globular | Lymphocytic infiltrate | ALect2 | + |
| 32 | 52 | F | Hispanic | Elevated LFTs | Portal/globular | Steatohepatitis | ALect2 | + |
| Case . | Age (y) . | Sex . | Ethnicity . | Presentation . | Histological pattern . | Other pathology . | MS results . | LECT2 IHC . |
|---|---|---|---|---|---|---|---|---|
| 1 | 65 | M | Caucasian | N/A | Portal/globular | Steatohepatitis | ALect2 | + |
| 2 | 41 | M | Caucasian | N/A | Portal/globular | Chronic hepatitis C | ALect2 | + |
| 3 | 60 | M | Caucasian | Incidental* | Portal/globular | Chronic hepatitis C | ALect2 | + |
| 4 | 70 | F | Hispanic | Cirrhosis, ascites, hepatitis | Portal/globular | Chronic hepatitis unknown type | ALect2 | + |
| 5 | 52 | F | Hispanic | Elevated LFTs | Portal/globular | Steatohepatitis | ALect2 | + |
| 6 | 65 | M | Hispanic | Elevated LFTs | Portal/globular | - | ALect2 | + |
| 7 | 76 | M | Hispanic | N/A | Portal/globular | - | ALect2 | + |
| 8 | 65 | F | Hispanic | Incidental* | Portal/globular | Steatosis | ALect2 | + |
| 9 | 62 | F | Hispanic | Incidental* | Portal/globular | Lymphocytic infiltrate | ALect2 | + |
| 10 | 58 | F | Caucasian | Incidental* | Portal/nodular | - | ALect2 | + |
| 11 | 62 | F | Hispanic | Elevated LFTs | Portal/globular | - | ALect2 | + |
| 12 | 36 | M | Hispanic | Elevated LFTs | Portal/globular | Lymphocytic infiltrate | ALect2 | + |
| 13 | 72 | F | Hispanic | Incidental* | Portal/nodular | - | ALect2 | + |
| 14 | 60 | F | Hispanic | N/A | Portal/globular | Lymphocytes infiltrate | ALect2 | + |
| 15 | 61 | F | Hispanic | Elevated LFTs | Portal/globular | Steatosis | ALect2 | + |
| 16 | 52 | F | Hispanic | Incidental* | Portal/globular | Cirrhosis, steatohepatitis | ALect2 | + |
| 17 | 79 | F | Hispanic | Elevated LFTs | Portal/globular | - | ALect2 | + |
| 18 | 63 | M | Hispanic | Elevated LFTs | Portal/globular | Cirrhosis | ALect2 | + |
| 19 | 54 | F | Hispanic | Elevated LFTs | Portal/globular | Steatosis, lymphocytic infiltrate | ALect2 | + |
| 20 | 74 | F | Hispanic | Incidental* | Portal/globular | Cirrhosis | ALect2 | + |
| 21 | 59 | M | Hispanic | N/A | Portal/globular | - | ALect2 | + |
| 22 | 61 | M | Hispanic | N/A | Portal/globular | - | ALect2 | + |
| 23 | 69 | M | Hispanic | N/A | Portal/globular | Chronic hepatitis C, portal fibrosis | ALect2 | + |
| 24 | 59 | M | Hispanic | N/A | Portal/globular | Lymphocytic infiltrate | ALect2 | + |
| 25 | 52 | F | Hispanic | N/A | Portal/globular | - | ALect2 | + |
| 26 | 53 | F | Hispanic | N/A | Portal/globular | - | ALect2 | + |
| 27 | 79 | F | Hispanic | N/A | Portal/globular | - | ALect2 | + |
| 28 | 66 | F | Hispanic | N/A | Portal/globular | Lymphocytic infiltrate | ALect2 | + |
| 29 | 33 | F | Hispanic | N/A | Portal/globular | Steatosis | ALect2 | + |
| 30 | 52 | M | Hispanic | Portal hypertension | Portal/globular | Steatohepatitis | ALect2 | + |
| 31 | 59 | F | Hispanic | Elevated LFTs | Portal/globular | Lymphocytic infiltrate | ALect2 | + |
| 32 | 52 | F | Hispanic | Elevated LFTs | Portal/globular | Steatohepatitis | ALect2 | + |
LFT: Liver function tests; N/A: data not available.
Liver biopsy performed during surgery for other diseases (cholecystectomy in 3 cases, allograft biopsy during laparotomy, and small bowel resection for partial obstruction caused by adhesions in 1 case, and biopsy of benign liver cysts in 1 case), or liver transplantation for hepatocellular carcinoma/end-stage liver disease (1 allograft liver biopsy and 1 explanted liver biopsy).
The pathological features of hepatic ALect2 amyloidosis were unique. All ALect2-involved liver specimens contained unusual globular amyloid deposits located in the periportal parenchyma or at the periphery of the portal triad, and sometimes around central venules (Figure 1B-C). This pattern was described previously,13-15 but its significance was unclear. Recognition of the characteristic globular pattern of ALect2 amyloid deposition helps one to consider ALect2 amyloidosis in the differential diagnosis of hepatic amyloidosis because it contrasts distinctly with the perisinusoidal amyloid deposition pattern that typifies hepatic AL amyloidosis.
Pathogenesis of hepatic ALect2 amyloidosis
LECT2 is synthesized principally by the liver, and is secreted by hepatoma cell lines.16-19 LECT2 serum levels are elevated in hepatic disorders.20-22 A cytokine is involved in the regulation of hepatocyte activity and functions as an immunomodulator.23 A polymorphism (Val58Ile) in the LECT2 gene (LECT2) was demonstrated to be a risk factor for the progression of rheumatoid arthritis.24
We investigated the expression of LECT2 protein and mRNA in hepatic amyloidosis and in normal and neoplastic tissues (supplemental Table 2). ALect2 amyloid deposits (Figure 1D) were stained positively by IHC. But normal liver, hepatocytes in ALect2 and other types of hepatic amyloidosis, and other normal and neoplastic tissues including hepatocellular carcinomas were negative. Because LECT2 is a cytokine rapidly secreted from the cell, we developed an ISH assay to analyze LECT2 mRNA expression. LECT2 mRNA was strongly and uniformly expressed in hepatocytes in ALect2 amyloidosis. Hepatocytes in normal liver and AL amyloidosis were negative or exhibited weak/focal LECT2 mRNA expression and there was variable expression in hepatocellular carcinoma. (Figure 1E-G and supplemental Table 2).
Because there was ethnic bias in ALect2 amyloidosis, we investigated the possibility of mutations affecting LECT2 in 1 patient with hepatic and 9 patients with renal ALect2 amyloidosis. We detected no mutations affecting the coding region of LECT2 in any of the patients. However, all cases were homozygous for the polymorphism in exon 3 (SNP rs31517 at the first position of codon 58, position 40 of the mature protein causing a change from valine to isoleucine) previously reported in renal ALect2 amyloidosis.9 Because this polymorphism is quite frequent (heterozygosity 0.472, minor allelic frequency 0.3732), it is unlikely to be the primary cause of ALect2 amyloidosis. Therefore, upregulation of LECT2 expression, either constitutively (controlled by yet undefined genetic factors) or secondary to hepatocellular damage is hypothesized to be the most likely cause of ALect2 amyloidosis.
Clinical significance of hepatic ALect2 amyloidosis
Our results show the value of untargeted methodologies such as LMD/MS to determine amyloid type in the liver. In addition to detecting previously known causes of hepatic amyloidosis, we showed that ALect2 amyloidosis accounted for 25% of hepatic amyloidosis cases. This is an important observation because prior to this study, ALect2 was not recognized as a cause of hepatic amyloidosis. These cases may be misdiagnosed as AL or AA amyloidosis based on the clinical context. Clinical management implications of such a mistake are significant. Therefore, accurate typing of amyloid deposits is essential before any management decision is made for hepatic amyloidosis patients. Given the characteristic demographic, histological, immunophenotypic, and proteomic features of ALect2 amyloidosis described in this study, recognition, and accurate diagnosis of hepatic ALect2 amyloidosis should be within the capabilities of most pathology laboratories.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Part of the methodology for LMD/MS analysis was performed with permission granted by OncoPlex Diagnostics, Rockville, MD.
O.M.M. is a visiting fellow from Center of Research on Immunopathology and Rare Diseases, S. Giovanni Bosco Hospital, University of Torino, Torino, Italy.
Authorship
Contribution: A. Dogan, O.M.M., J.D.T., and J.A.V. designed the study; O.M.M., J.D.T., J.A.V., M.E.L., S.D., K.L.G., P.J.K., and A. Dogan performed experiments and analyzed data; V.S.C., T.T.W., V.H.J.Z., R.F., and A. Dispenzieri provided patient data; and O.M.M., P.J.K., and A. Dogan wrote the manuscript with contributions from all other authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A. Dogan is Memorial Sloan-Kettering Cancer Center, New York, NY.
Correspondence: Ahmet Dogan, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: dogana@mskcc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal