Key Points
Antisense oligonucleotides reduce levels of target hepatic mRNA and protein and decrease clotting activity in rabbits.
Selective depletion of factors XI and XII in rabbits attenuates catheter thrombosis, whereas factor VII depletion does not.
Abstract
Central venous catheter thrombosis can cause venous obstruction and pulmonary embolism. To determine the extent to which catheter thrombosis is triggered by the contact or extrinsic pathway of coagulation, we used antisense oligonucleotides (ASOs) to selectively knock down factor (f)XII, fXI, or high-molecular-weight kininogen (HK), key components of the contact pathway, or fVII, which is essential for the extrinsic pathway. Knockdown of contact pathway components prolonged the activated partial thromboplastin time and decreased target protein activity levels by over 90%, whereas fVII knockdown prolonged the prothrombin time and reduced fVII activity to a similar extent. Using a rabbit model of catheter thrombosis, catheters implanted in the jugular vein were assessed daily until they occluded, up to a maximum of 35 days. Compared with control, fXII and fXI ASO treatment prolonged the time to catheter occlusion by 2.2- and 2.3-fold, respectively. In contrast, both HK and fVII knockdown did not significantly prolong the time to occlusion, and dual treatment with fVII- and fXI-directed ASOs produced a time to occlusion similar to that with the fXI ASO alone. These findings suggest that catheter thrombosis is triggered via the contact pathway and identify fXII and fXI as potential targets to attenuate this complication.
Introduction
Central venous catheters (CVCs) are frequently used in patients with cancer, including those with hematologic malignancies. Patients with cancer are at risk for thrombosis, and indwelling CVCs increase this risk.1-3 Thrombosis associated with CVCs can cause upper-extremity deep-vein thrombosis, which can lead to pulmonary embolism. With more extensive thrombosis, superior vena cava syndrome can occur.4 Symptomatic thrombosis occurs in at least 5% of cancer patients with CVCs and is a serious problem because it often delays cancer treatment, prolongs hospital stay, and increases health care costs by necessitating anticoagulant therapy in patients at risk for bleeding.4
The pathogenesis of thrombosis in patients with CVCs is unclear. Low doses of warfarin and prophylactic low-molecular-weight heparin do not reduce the risk of CVC-associated thrombosis.5 The failure of these agents to prevent this problem highlights the need for a better understanding of the mechanism of catheter thrombosis so that more targeted preventive therapy can be developed.
According to the classic waterfall model of blood coagulation, coagulation can be triggered by the tissue factor-factor (f)VIIa complex, which initiates the extrinsic pathway, or by fXIIa, which initiates the contact pathway. Although evidence in humans and studies in mice have confirmed the role of the tissue factor pathway in hemostasis and thrombosis,6 the contact system plays no part in hemostasis and its role in thrombosis is uncertain.7 fXII is activated in the presence of high-molecular-weight kininogen (HK) and goes on to activate fXI and prekallikrein. Historically, artificial surfaces and, more recently, native polyanionic compounds such as nucleic acids and inorganic polyphosphates have been identified as potential cofactors in fXII activation.8-10 Recently, we showed that (1) catheters have prothrombotic activity in plasma and initiate clotting by activating fXII and (2) corn trypsin inhibitor, a potent inhibitor of fXIIa, attenuates catheter-induced clotting.11,12 If the same is true in vivo, we hypothesized that the use of liver-directed antisense oligonucleotides (ASOs) to selectively knock down contact pathway factors fXII, fXI, and HK in rabbits would attenuate catheter thrombosis, whereas fVII knockdown would have little or no effect. To further explore the involvement of the extrinsic pathway in catheter-induced clotting, we also examined the effect of combined knockdown of fVII plus fXI.
Methods
Materials
Solo-Cath polyurethane single-lumen catheters (PU-C70, 7F × 15 cm) with slightly rounded distal tips, integrated Luer locks, and suture flanges were purchased from Solomon Scientific (Plymouth Meeting, PA). Goat immunoglobulin G (IgG) directed against human fXII and fXI, peroxidase-conjugated sheep IgG directed against human HK, and fVII-, fXI-, fXII-, and HK-deficient human plasma were purchased from Affinity Biologicals (Ancaster, ON, Canada). Goat antibodies directed against fXII and fXI were conjugated to horseradish peroxidase using Lightning Link (Innova Biosciences, Cambridge, United Kingdom). To prepare a pool of normal rabbit plasma, blood collected from at least 5 healthy rabbits into 3.8% sodium citrate was subjected to centrifugation and the resultant platelet poor plasma was harvested, pooled, and frozen in aliquots at −70°C as described previously.11
Preparation of ASOs
The methods for synthesis and purification of the control, fVII-, fXI-, fXII-, and HK-directed phosphorothioate ASOs are described in the supplemental Methods on the Blood Web site. The primer probe sets used for synthesis are listed in supplemental Table 1.
Dosing of ASOs in rabbits
Male New Zealand white rabbits (2.5-3.0 kg), purchased from Charles River Canada (Sherbrooke, QC, Canada), were housed in individual cages in rooms maintained on a constant 12-hour light-dark cycle with controlled temperature and humidity and were given free access to food and water. Studies were approved by the Animal Research Ethics Board at McMaster University, and procedures complied with Canadian Council on Animal Care guidelines.
After identifying the ASO sequences that produced the greatest reductions in factor levels in a preliminary study (described in the supplemental Methods), 5 groups of rabbits (n = 7-12 per group) were randomized to receive subcutaneous injections of control, fVII-, fXI-, fXII-, or HK-directed ASOs at a dose of 15 mg/kg twice weekly (Table 1). For combined treatment, rabbits received 15-mg/kg twice-weekly subcutaneous injections of both fVII- and fXI-directed ASOs. The control ASO consisted of a scrambled oligonucleotide.13 All treatments were given for 4 weeks prior to catheter implantation and continued for 5 weeks thereafter. During treatment, rabbits were monitored daily for signs of toxicity and body weight was recorded weekly. At the end of the treatment period, we examined the effect of the ASOs on clotting factor hepatic messenger RNA (mRNA) expression, protein levels as determined by immunoassay and by clotting activity using appropriate factor-deficient human plasma, and global tests of coagulation. Investigators performing the rabbit studies and conducting the sample analyses were blinded as to treatment allocation.
ASOs directed against rabbit coagulation factors
| ISIS # . | Target . | Sequence . |
|---|---|---|
| 141923 | Control | CCTTCCCTGAAGGTTCCTCC |
| 608032 | Factor VII | CTGCAAGTGTCTCTCCCCTT |
| 564673 | Factor XI | GTAACATGTGCCCTTTCCTT |
| 564859 | Factor XII | GGAATGGCCATTGTCCTCGC |
| 567518 | HK | GCTATTCTGAGACATCATGG |
| ISIS # . | Target . | Sequence . |
|---|---|---|
| 141923 | Control | CCTTCCCTGAAGGTTCCTCC |
| 608032 | Factor VII | CTGCAAGTGTCTCTCCCCTT |
| 564673 | Factor XI | GTAACATGTGCCCTTTCCTT |
| 564859 | Factor XII | GGAATGGCCATTGTCCTCGC |
| 567518 | HK | GCTATTCTGAGACATCATGG |
Preparation of platelet-poor rabbit plasma
Blood samples were collected under anesthesia every week until study termination. Blood (4 mL) was withdrawn from a central ear artery catheter using a 5-mL syringe containing 0.5 mL of 3.8% sodium citrate. Samples were immediately mixed and stored at 4°C prior to centrifugation for 15 minutes at 2000g at 23°C. Plasma was subjected to a second centrifugation step under the same conditions, pooled, and frozen in aliquots at −70°C.
Hepatic fVII, fXI, fXII, and HK mRNA expression
To assess mRNA expression, a 0.5-cm3 section of liver collected from each rabbit at postmortem examination was submerged in RNALater solution (Life Technologies, Burlington, ON), stored overnight at 4°C, and then frozen at −70°C. After homogenization of thawed samples, mRNA was isolated using the PureLink Pro 96 Total RNA Purification Kit (Life Technologies) and fVII, fXI, fXII, and HK mRNA levels were quantified using OneStepPlus real-time PCR (Applied Biosystems, Foster City, CA) and normalized against a rabbit 18S ribosomal RNA primer probe set.14
Immunoblot analysis
Plasma was subjected to electrophoresis on SDS 4% to 15% polyacrylamide gradient gels (Bio-Rad Laboratories, Hercules, CA) under nonreducing conditions and separated proteins were then transferred to Immuno-Blot polyvinylidene fluoride membranes (Bio-Rad) as described elsewhere.15 Clotting factors were detected by immunoblot analysis using horseradish-peroxidase–conjugated IgG directed against human fVII, fXI, fXII, or HK. Blots were incubated with Immuno-Star Western reagent (Bio-Rad) and imaged on a ChemiDoc XRS+ System using Image Laboratory, v3.0 software (Bio-Rad), and protein levels were then quantified by densitometry.
Global tests of coagulation
Activated partial thromboplastin time (aPTT) and dilute prothrombin time (PT) measurements were obtained using a SpectroMax 340PC384 plate reader (Molecular Devices, Sunnyvale, CA). For aPTT determination, 50 µL of platelet-poor rabbit plasma was incubated with 50 µL of aPTT reagent (APTT-SP HemosIL; Instrumentation Laboratory, Bedford, MA) for 5 minutes at 37°C, followed by recalcification with 50 µL of 25 mM CaCl2. For PT determination, 50 µL of platelet-poor rabbit plasma was incubated for 2 minutes at 37°C prior to addition of 100 µL of a 1/100 dilution of RecombiPlastin, a recombinant tissue factor (Instrumentation Laboratory), containing 25 mM CaCl2. Clotting was monitored by measuring absorbance at 405 nm with a plate reader, and clotting times were recorded as the time to half-maximum absorbance by instrument software (SoftMax Pro, v5.4).
Clotting factor protein activity
Functional plasma levels of fVII, fXI, fXII, and HK were quantified by clotting assay using the appropriate factor-deficient human plasma. Briefly, platelet-poor rabbit plasma was diluted 1:20 in 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (pH 7.4) and 150 mM NaCl buffer containing 0.1% (weight/volume) bovine serum albumin. In a 96-well plate, 30 µL of diluted plasma was incubated with 30 µL APTT-SP and 30 µL of citrated human plasma deficient in fXI, fXII, or HK for 5 minutes at 37°C. A similar system was used to quantify levels of fVII except RecombiPlastin was used in place of aPTT reagent. In all cases, clotting was initiated by addition of 30 µL of a 25-mM CaCl2 solution, and clot formation was assessed by monitoring absorbance at 340 nm in kinetic mode using a plate reader. Clotting times were taken as the time to achieve half-maximal increase in absorbance as determined by the instrument software. Activity levels of fVII, fXI, fXII, or HK were interpolated from standard curves prepared using serial dilutions of citrated normal rabbit reference plasma and expressed as a percentage of normal.
Rabbit model of catheter thrombosis
The rabbit model of catheter thrombosis was a modification of that described by Klement et al.16 Rabbits that had received control, fVII, fXI, fXII, or HK ASOs for 4 weeks as described above were sedated using a ketamine/xylazine mixture. The hair over the right craniolateral neck and right ear was clipped and the skin was prepared for sterile surgery. Rabbits were then given inhalational anesthesia consisting of oxygen and 3% to 5% isoflurane via a mask. Catheters were flushed inside and outside with 10 mL of sterile normal saline followed by a final flush with 1 mL of saline to remove any air bubbles. Under sterile conditions, a 2-cm skin incision was made to isolate the right external jugular vein. A subcutaneous tunnel was then created from the incision site to the posterior base of the auricular cartilage. After incising the auricular cartilage and overlying skin to facilitate retrograde passage, the proximal portion of the catheter was secured to the skin of the external auditory meatus using a single 4-0 Prolene cruciate ligature. The jugular vein was ligated proximally and, under distal occlusion, the catheter was introduced into the vein and advanced into the superior vena cava. After inserting 7 cm of catheter, the catheter was anchored at the insertion site with 2 4-0 Vicryl sutures. Skin incisions were closed with stainless-steel wound clips. Any blood loss was replaced with an equivalent volume of intravenous saline, and buprenorphine and enrofloxacin (0.02 and 10 mg/kg, respectively) were administered intramuscularly for pain and infection control, respectively.
After recovery, rabbits were returned to their cages and monitored daily. Catheter patency was examined daily by withdrawing 0.5 mL of blood and then flushing the catheter with 2 mL of saline. A pressure transducer (Baxter Healthcare), placed between the catheter and the syringe, was used to quantify pressure within the catheter. Catheter occlusion was taken as the time when blood could no longer be withdrawn, saline could no longer be flushed, and the pressure within the catheter was >100 mm Hg during the saline flush. At this point, or at 35 days if occlusion did not occur, the study was terminated, as described elsewhere.11,16
Statistical analyses
Results are presented as mean ± standard deviation (SD). Unless otherwise stated, experiments were performed at least 3 times. Means of paired data were compared by analysis of variance followed by post hoc analysis using Tukey’s test. For all analyses, P values < .05 were considered statistically significant.
Results
Effect of ASO-mediated knockdown on mRNA expression and clotting factor levels
After 4 weeks of treatment, the fVII-, fXI-, fXII-, and HK-directed ASOs significantly (P < .001) reduced respective mRNA expression by 92%, 84%, 97%, and 57% (Figure 1A). Coincident with reduced mRNA expression, plasma levels of fXI, fXII, and HK, as detected by immunoblot analysis, also were significantly (P < .001) reduced by 96%, 97%, and 87%, respectively (Figure 1B). The HK-directed ASO not only decreased plasma HK but also significantly (P < .05) decreased the plasma level of fXI by 76%. Plasma fVII protein levels could not be determined immunologically because there are no commercially available antibodies directed against rabbit fVII and human and mouse fVII-directed antibodies exhibit poor cross-reactivity (data not shown). In functional assays, the respective ASOs significantly (P < .001) reduced fVII, fXI, fXII, and HK activity by 92%, 99%, 99%, and 91%, respectively (Figure 1C). In addition to lowering HK activity, the HK directed ASO also significantly (P < .001) decreased fXI activity by 72%. Thus, the ASOs decrease target mRNA and protein levels, as well as plasma clotting activity.
Effect of fVII, fXI, fXII, and HK ASOs on hepatic mRNA expression, protein levels, and activity. Male New Zealand white rabbits were treated subcutaneously with control, fVII, fXI, fXII, and HK ASO for 4 weeks at 15-mg/kg twice-weekly dose (n = 8 per treatment group). Two days after final dosing, blood was collected for quantification of (A) hepatic fVII, fXI, fXII, and HK mRNA expression, (B) fXI, fXII, and HK protein levels by immunoblot analysis, and (C) procoagulant activity in fVII- (black bars), fXI- (dark gray bars), fXII- (light gray bars), and HK-deficient (white bars) human plasma. The bars for mRNA and activity levels represent the mean of 3 separate experiments for each rabbit, whereas the lines above the bars reflect the SD. *P < .05 compared with control ASO.
Effect of fVII, fXI, fXII, and HK ASOs on hepatic mRNA expression, protein levels, and activity. Male New Zealand white rabbits were treated subcutaneously with control, fVII, fXI, fXII, and HK ASO for 4 weeks at 15-mg/kg twice-weekly dose (n = 8 per treatment group). Two days after final dosing, blood was collected for quantification of (A) hepatic fVII, fXI, fXII, and HK mRNA expression, (B) fXI, fXII, and HK protein levels by immunoblot analysis, and (C) procoagulant activity in fVII- (black bars), fXI- (dark gray bars), fXII- (light gray bars), and HK-deficient (white bars) human plasma. The bars for mRNA and activity levels represent the mean of 3 separate experiments for each rabbit, whereas the lines above the bars reflect the SD. *P < .05 compared with control ASO.
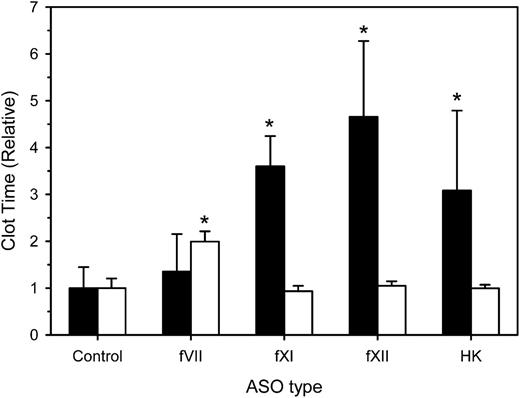
Effect of ASOs on global tests of coagulation
We next examined the effect of factor depletion on the aPTT and dilute PT at 4 weeks. Compared with a mean aPTT of 258 ± 115 seconds with control ASO, the mean aPTT values in rabbits treated with fXI, fXII, and HK-directed ASOs were significantly prolonged by 3.1- (P = .009), 4.7- (P < .001), and 3.6-fold (P < .001), whereas the fVII-directed ASO had no effect (Figure 2). The mean PT in rabbits given control ASO was 130 ± 10 seconds. As expected, mean PT values in rabbits treated with fXI-, fXII-, and HK-directed ASOs were similar to that in controls. In contrast, the mean PT in rabbits given fVII-directed ASO was prolonged twofold (P < .001). Taken together, these results indicate that contact-factor–directed ASOs prolong the aPTT, whereas the fVII-directed ASO prolongs the PT.
Effect of treatment with control, fVII, fXI, fXII, or HK ASOs on the aPTT and dilute PT. Rabbits were treated subcutaneously with control, fVII, fXI, fXII, or HK ASOs for 4 weeks at 15 mg/kg twice weekly (n = 8 per treatment group). Two days after the last ASO dose, blood was collected for determination of the aPTT (black bars) or dilute PT (white bars). Values were normalized relative to those obtained in rabbits given the control ASO. The bars represent the mean of 3 separate determinations for each rabbit, whereas the lines above the bars reflect the SD. *P < .05 compared with control ASO.
Effect of treatment with control, fVII, fXI, fXII, or HK ASOs on the aPTT and dilute PT. Rabbits were treated subcutaneously with control, fVII, fXI, fXII, or HK ASOs for 4 weeks at 15 mg/kg twice weekly (n = 8 per treatment group). Two days after the last ASO dose, blood was collected for determination of the aPTT (black bars) or dilute PT (white bars). Values were normalized relative to those obtained in rabbits given the control ASO. The bars represent the mean of 3 separate determinations for each rabbit, whereas the lines above the bars reflect the SD. *P < .05 compared with control ASO.
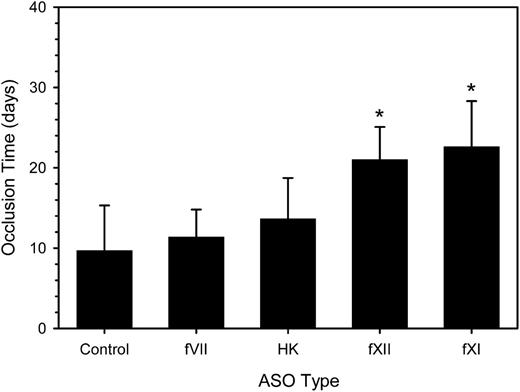
Effects of fXI, fXII, and HK ASO treatment on catheter patency in rabbits
Having demonstrated that a 4-week period of ASO treatment reduced target factor activity, we explored the relative importance of fVII, fXI, fXII, and HK on catheter patency over a subsequent 35-day period. Treatment with twice-weekly injections of ASOs or control was continued during this period. With control ASO, the time to catheter occlusion was 9.7 ± 5.6 days (Figure 3), a value similar to the median number of days between catheter insertion and catheter thrombosis in cancer patients.17 Treatment with fXI or fXII ASO significantly prolonged the mean time to catheter occlusion by 2.3- and 2.2-fold, respectively (P < .001). In contrast, treatment with HK ASO only prolonged the mean time to catheter occlusion 1.4-fold (P = .97), even though this regimen also decreased fXI activity by 72% (Figure 1). Likewise, treatment with fVII ASO produced a nonsignificant 1.2-fold prolongation in the mean time to catheter occlusion. Thus, we demonstrated that treatment with fXI and fXII ASO, but not HK or fVII ASO, attenuates the prothrombotic activity of catheters in rabbits.
Effect of fVII, HK, fXII, and fXI ASO treatment on the time to catheter occlusion. Rabbits (n = 7-12 per group) were given a 4-week course of control ASO or fVII, HK, fXII, or fXI ASO prior to insertion of a catheter into their jugular veins and treatment was continued. Every day for 35 days, 0.5 mL of blood was withdrawn from the catheter into a syringe and slowly reinjected. The catheter was then flushed 2 mL of saline. Catheter occlusion occurred when blood could no longer be withdrawn, saline could no longer be injected, and the pressure measured with a transducer exceeded 100 mm Hg. The bars represent the mean of at least 7 separate experiments, whereas the lines above the bars reflect the SD. *P < .001 compared with the control.
Effect of fVII, HK, fXII, and fXI ASO treatment on the time to catheter occlusion. Rabbits (n = 7-12 per group) were given a 4-week course of control ASO or fVII, HK, fXII, or fXI ASO prior to insertion of a catheter into their jugular veins and treatment was continued. Every day for 35 days, 0.5 mL of blood was withdrawn from the catheter into a syringe and slowly reinjected. The catheter was then flushed 2 mL of saline. Catheter occlusion occurred when blood could no longer be withdrawn, saline could no longer be injected, and the pressure measured with a transducer exceeded 100 mm Hg. The bars represent the mean of at least 7 separate experiments, whereas the lines above the bars reflect the SD. *P < .001 compared with the control.
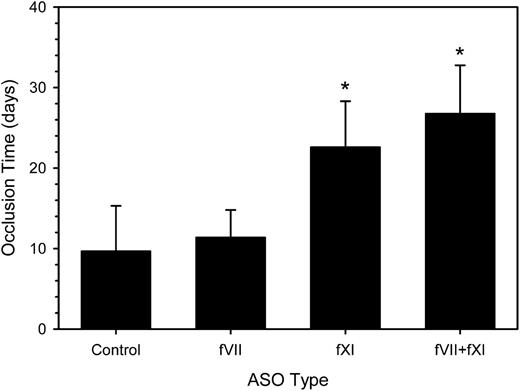
To further investigate the role of fVII in catheter thrombosis, we coadministered fVII- and fXI-directed ASOs. The combination produced a 2.4-fold prolongation of the aPTT (P < .001) and 1.5-fold prolongation of the PT (P = .003) compared with control ASO (data not shown). The mean time to catheter occlusion was prolonged by 2.8-fold (P < .001), a value significantly longer than that with the fVII ASO alone (P < .001), but not significantly different from that with the fXI ASO alone (P = .55) (Figure 4). Thus, even in the absence of a functional contact system, the extrinsic pathway does not appear to play a part in catheter-induced clotting in this model.
Effect of fVII and/or fXI ASO treatment on the time to catheter occlusion. Rabbits (n = 8-12 per group) were given a 4-week course of control ASO, fVII or fXI ASO, or fVII plus fXI ASO combination subcutaneously prior to insertion of a catheter into their jugular veins and treatment was continued. Catheter occlusion was assessed as described. The bars represent the mean of at least 8 separate experiments, whereas the lines above the bars reflect the SD. *P < .001 compared with the control.
Effect of fVII and/or fXI ASO treatment on the time to catheter occlusion. Rabbits (n = 8-12 per group) were given a 4-week course of control ASO, fVII or fXI ASO, or fVII plus fXI ASO combination subcutaneously prior to insertion of a catheter into their jugular veins and treatment was continued. Catheter occlusion was assessed as described. The bars represent the mean of at least 8 separate experiments, whereas the lines above the bars reflect the SD. *P < .001 compared with the control.
Discussion
The purpose of this study was to delineate the relative contributions of the contact and extrinsic pathway to catheter thrombosis by targeted clotting factor knockdown using ASO technology. After confirming that ASOs directed against fVII, fXI, fXII, and HK reduced respective rabbit liver mRNA, plasma protein, and coagulant activity levels, we showed that selective knockdown of fXI, fXII, or HK prolonged the aPTT, whereas selective knockdown of fVII prolonged the PT. Neither treatment regimen affected the function of the other pathway. In the rabbit model of catheter thrombosis, the time to catheter occlusion was prolonged with knockdown of fXI and fXII, but not with knockdown of fVII or HK. These findings (1) suggest that catheter thrombosis is triggered via the contact pathway and that the extrinsic pathway plays little or no role in this process and (2) identify fXI and fXII as potential targets to attenuate catheter thrombosis.
Recent studies using ASO technology have identified roles for fXI, fXII, and prekallikrein in arterial and venous thrombosis in mice and nonhuman primates.18-21 Because ASOs are species specific, we developed ASOs that target rabbit coagulation factors. Consistent with previous investigations in other species, fXI- and fXII-directed ASO treatment reduced mRNA, protein expression, and procoagulant activity in a targeted fashion. Importantly, fXI- and fXII-directed ASO treatment bestowed an antithrombotic phenotype in rabbits, as shown by the prolongation of the time to occlusion. Therefore, fXII-, fXI-, HK-, and fVII-directed ASOs can be added to the list of effective ASOs for study in rabbits, and these can be used to examine the effect of contact factor and fVII knockdown in models of thrombosis.
Treatment with HK-directed ASO not only reduced the level of HK but also was associated with a concomitant reduction in the level of fXI despite normal hepatic fXI mRNA expression. This finding raises the possibility that HK modulates fXI clearance, an observation that deserves further exploration. Unlike fXI or fXII knockdown, HK knockdown did not prolong the time to catheter occlusion. There are several potential explanations. First, although plasma levels of HK were reduced by 87%, the residual HK may have been sufficient to amplify contact activation, because over 90% HK depletion is required to prolong clotting times in plasma.22 Second, although HK accelerates fXI activation by fXIIa, the reaction can occur in the absence of HK, albeit at a slower rate.23 Third, polyanions, such as inorganic polyphosphates,24 may substitute for HK. Lastly, the extent of fXI depletion may have been inadequate. Although HK ASO produced a 72% reduction, there may have been sufficient fXI to induce clotting. Any or all of these phenomena may explain why fXI and fXII knockdown attenuated catheter-induced clotting in rabbits more than HK knockdown.
There is abundant evidence that fXII is activated by negatively charged surfaces such as glass, kaolin, dextran sulfate, sulfatides, and polymers.25-29 However, the role of the contact pathway in thrombosis induced by catheters or other blood-contacting devices has been a matter of debate.30,31 Likewise, although the extrinsic pathway is essential for hemostasis, its contribution to catheter thrombosis also is uncertain.30,31 In this study, we show that knockdown of fXI or fXII prolongs the time to catheter occlusion, whereas fVII knockdown does not, nor does concomitant knockdown of fVII and fXI extend the time to catheter occlusion beyond that produced by fXI knockdown alone. These findings suggest that catheter thrombosis is mainly driven by the contact pathway and that the extrinsic pathway does not play a major role in this process. Although the small amount of circulating fVII that remains after fVII knockdown in rabbits may be sufficient to trigger the extrinsic pathway, this is unlikely to explain why fVII knockdown had little effect on the time to catheter occlusion for at least two reasons. First, consistent with the findings in rabbits, we showed that catheter-induced clotting in vitro is attenuated in plasma deficient in fXI or fXII, but not in plasma deficient in fVII.11 Second, there was more catheter thrombosis with nematode anticoagulant protein (NAP) c2, a potent inhibitor of fVIIa, than with heparin when the agents were compared in a phase 2 study in patients with acute coronary syndrome,32 a finding that suggests that extrinsic pathway inhibition fails to prevent catheter thrombosis. Therefore, catheter thrombosis appears to be triggered by the contact pathway.
Selective knockdown of fXI and fXII in rabbits conferred protection against catheter-induced occlusion, findings in keeping with our earlier observation that catheter-induced clotting is attenuated to a similar extent in fXI- and fXII-deficient human plasma.11 Ferric-chloride–induced thrombosis is attenuated in mice deficient in fXI or fXII and when fXI or fXII is knocked down with ASOs,19,33,34 supporting the role of fXI and fXII in thrombosis. Together, these results confirm the role of fXI and fXII in thrombosis and validate fXI and fXII as novel targets for antithrombotic therapy.
Targeting the contact pathway offers potential benefits over conventional anticoagulant therapies. Although the delayed knockdown with ASOs limits their utility in the acute setting, inhibitory antibodies against fXIa35 and fXII,36 small-molecule inhibitors of fXIa,37 inhibitory nanobodies against fXIIa,38 and RNA aptamers targeting fXII39 offer promise for the future. Alternatively, surface modification using CTI, an fXIIa inhibitor, provides a method for rendering catheters and other blood-contacting devices less thrombogenic.12,40-42 The utility of these agents for the prevention and treatment of catheter thrombosis requires further investigation.
In conclusion, using targeted ASO knockdown in rabbits, we provide evidence that catheter thrombosis is triggered via the contact pathway. Furthermore, our studies identify fXI and fXII as potential targets to attenuate catheter thrombosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Shawn Petrik for constructive comments on the paper.
This work was supported in part by grants from the Canadian Institutes of Health Research (FRN 3992, MOP 102735, and FRN 79846) and the Heart and Stroke Foundation of Ontario (T4729 and T4730). J.I.W. is the recipient of the Heart and Stroke Foundation of Ontario/J. Fraser Mustard Endowed Chair in Cardiovascular Research and Canada Research Chair (Tier 1) in Thrombosis at McMaster University.
Authorship
Contribution: J.W.Y. designed research, performed research, analyzed and interpreted data, and wrote the manuscript; P.L. performed research and analyzed and interpreted data; J.C.F. designed research, analyzed and interpreted data, and wrote the manuscript; A.R.S. performed research; A.S.R. and B.P.M. synthesized ASOs, performed research, and analyzed and interpreted data; and J.I.W. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: A.S.R. and B.P.M. are employees of Isis Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Jeffrey I. Weitz, Thrombosis and Atherosclerosis Research Institute, 237 Barton St E, Hamilton, ON L8L 2X2, Canada; e-mail: weitzj@taari.ca.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal