Abstract
Lymphatic vasculature is increasingly recognized as an important factor both in the regulation of normal tissue homeostasis and immune response and in many diseases, such as inflammation, cancer, obesity, and hypertension. In the last few years, in addition to the central role of vascular endothelial growth factor (VEGF)-C/VEGF receptor-3 signaling in lymphangiogenesis, significant new insights were obtained about Notch, transforming growth factor β/bone morphogenetic protein, Ras, mitogen-activated protein kinase, phosphatidylinositol 3 kinase, and Ca2+/calcineurin signaling pathways in the control of growth and remodeling of lymphatic vessels. An emerging picture of lymphangiogenic signaling is complex and in many ways distinct from the regulation of angiogenesis. This complexity provides new challenges, but also new opportunities for selective therapeutic targeting of lymphatic vasculature.
Overview of lymphatic vasculature
Lymphatic vessel structure and function
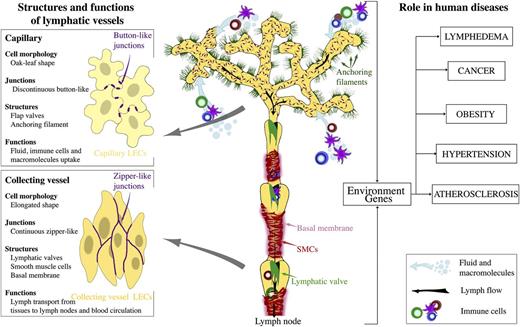
The vascular system consists of blood and lymphatic vessels, both of which are lined by endothelial cells. Lymphatic vasculature is composed of a branched network of capillaries and collecting lymphatic vessels, present in most organs (Figure 1).1 Unlike the blood vasculature, lymphatic capillaries are blind-ended. Small capillaries drain into precollecting and collecting vessels that join the thoracic duct. Lymph flows through collecting vessels and lymph nodes before ultimately being returned to venous circulation at junctions with the subclavian vein.
Organization and the role of lymphatic vasculature in physiology and human disease. Lymphatic capillaries uptake interstitial fluid, lipids, and proteins and serve as entry points to immune cells. Lymphatic collecting vessels transport the lymph toward lymph nodes and to blood circulation. Intraluminal lymphatic valves and SMCs coordinate lymph propulsion and the direction of flow.
Organization and the role of lymphatic vasculature in physiology and human disease. Lymphatic capillaries uptake interstitial fluid, lipids, and proteins and serve as entry points to immune cells. Lymphatic collecting vessels transport the lymph toward lymph nodes and to blood circulation. Intraluminal lymphatic valves and SMCs coordinate lymph propulsion and the direction of flow.
The specialized, discontinuous, “button-like” junctions between endothelial cells of lymphatic capillaries represent sites of fluid and immune cell entry into lymphatic vasculature.2 Unlike blood capillaries, lymphatic capillaries have a sparse basement membrane and lack pericytes. They are also attached to extracellular matrix by anchoring filaments. Collecting lymphatic vessels have continuous cell-cell junctions, and they are covered with basement membrane and smooth muscle cells (SMCs). Collecting lymphatic vessels contain intraluminal valves, which consist of 2 semilunar leaflets, covered by a specialized endothelium attached to the core of extracellular matrix.3 High lymph pressure upstream of a valve opens the valve and enables lymph flow, whereas flow in the reverse direction pushes leaflets against each other and closes the valve, depending on changes in fluid pressure within the collecting vessels. SMCs that cover the lymphangions contract and regulate the lymph flow.4
Main steps of lymphatic vascular development in mammals
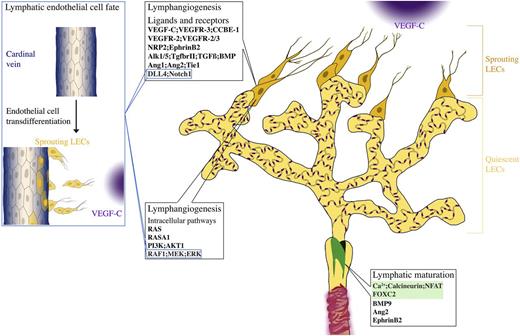
The early studies of lymphatic vasculature suggested the 2 potential sources of lymphatic endothelial cells (LECs) during development: blood vessels5 and mesenchymal cells.6 Recent studies, using high-resolution imaging of developing mouse embryos and lineage tracing approaches,7,8 suggest that the majority of LECs in mammals are generated through trans-differentiation from embryonic veins. LEC commitment requires Sry-related Hmg-box 18 (Sox18) and the orphan nuclear receptor chicken ovalbumin upstream promoter transcription factor (COUP-TFII), which up-regulate prospero-related homeodomain transcription factor (Prox1), essential for lymphatic endothelial-specific programming in mammals, but not zebrafish.9-11 Prox1 down-regulates blood markers and vascular endothelial growth factor receptor (VEGFR)-3, which is important for LEC survival, migration, and proliferation. LECs then start to bud by following the gradient of VEGFR-3 ligand, VEGF-C.1 In mice, LECs begin to sprout from the cardinal veins and intersomitic vessels around embryonic day (E)10.7-9 LECs emigrate from veins as nonlumenized, loosely connected strings of cells, so that the integrity of the blood vessel is not disturbed.7,8 LECs further coalesce to form 2 large primordial vessels: the dorsal peripheral longitudinal lymphatic vessel and the ventral primordial thoracic duct, which are also commonly called “lymph sacs” in earlier publications.7 The primordial thoracic duct establishes a connection with the cardinal vein to provide a path for the return of lymph into the blood circulation. The connection is protected by the lymphovenous valve, formed by a small subpopulation of LEC progenitors remaining in the veins,12 which is important for preventing the backflow of blood into the thoracic duct.
Embryonic lymphatic vasculature is established by further sprouting from the primordial lymphatic structures. During late gestation and the postnatal period, the lymphatic capillary plexus is remodeled to establish functional compartments of lymphatic vasculature, capillaries, and the collecting lymphatic vessels.1 During this process, collecting lymphatic vessels develop intraluminal valves and acquire SMCs and basement membrane coverage, whereas lymphatic capillaries remodel cell junctions from the continuous “zipper-like” to the discontinuous button-like state.1,13 Lymphangiogenesis and vessel remodeling continues in many organs, including skin and intestine during early postnatal development, postnatal mouse mammary gland morphogenesis,14 and ovarian follicle growth in adult mice.15
Lymphatic vessels in disease
In the adult tissues, the majority of lymphatic vessels are quiescent, with the exception of reproductive organs during the ovarian cycle and gestation15 ; however, reactivation of vessels occurs in a variety of pathological conditions. Such vessels may show loss of specialized junctions in capillaries, observed in inflammation,13 and ectopic recruitment of SMCs to lymphatic capillaries, such as in lymphedema.16
Lymphangiogenesis has been observed in many human inflammatory diseases, including psoriasis and rheumatoid arthritis, and during transplant rejection.17,18 Inflammation-induced lymphangiogenesis regulates fluid drainage, immune cell migration, and removal of inflammatory mediators, ultimately accelerating the resolution of inflammation.19 In contrast, following organ transplantation, enhanced lymphangiogenesis induces reactivation of immune system in the draining lymph node, which results in organ rejection.18 Inflammatory lymphangiogenesis has frequently been shown to occur via secretion of lymphangiogenic growth factors, such as Vegf-c, by macrophages.20 In addition, neutrophils are also recruited to sites of inflammation where they modulate lymphangiogenesis via Vegf-a bioavailability and, to a lesser extent, secretion of Vegf-d.21 Hence, stimulation or inhibition of lymphangiogenesis represents an attractive novel therapeutic strategy for reducing chronic inflammation or transplant rejection.
In cancer, lymph node status is an important factor in determining the stage of disease progression. Many cancers exploit lymphatic vasculature to spread to lymph nodes.22,23 Adding to the disease burden is the development of secondary lymphedema in patients who have undergone radical axillary lymph node dissection.24 Many cancer types induce lymphangiogenesis via release of VEGF-C or VEGF-D, and blocking VEGFR-3 signaling inhibits tumor lymphangiogenesis as well as lymph node metastasis in animal models.25 Increased lymphatic vessel density is correlated with a poor prognosis in a number of solid tumors.22 Intratumoral vessels are thought to be collapsed and nonfunctional due to the high intratumoral fluid pressure.26 In contrast, peritumoral lymphatics are often dilated containing clusters of tumor cells.27,28 15-Lipoxygenase-1-expressing tumor cells, which secrete arachidonic acid metabolites which help to invade lymphatic vessels by inducing the formation of holes in LEC plasma membrane.27 Dilation of collecting vessels through the action of tumor-produced VEGF-C and prostaglandins further contributes to cancer dissemination to lymph nodes.29,30 Lymphangiogenesis in sentinel lymph nodes even before tumors cells metastasize has been shown in mouse models,31 and the extent of lymph node lymphangiogenesis correlates with the disease prognosis in several human cancer types.32,33 Lymph node lymphatic sinuses, but not peripheral lymphatic vessels, express CC chemokine ligand (CCL)-1 chemokine, which regulates the entry of CC chemokine receptor (CCR)-8+ tumor cells into the lymph node.34
Lymphatic vessel dysfunction has been linked to obesity, and cardiovascular disease such as atherosclerosis. Impaired lymphatic function, such as in Prox1+/− mice, which have leaky lymphatic vessels, leads to adult-onset obesity and inflammation.35 Conversely, lymphatic function is impaired in obese patients.36 Lymphatic vasculature has a direct role in the reverse cholesterol transport, a process critical for the removal of cholesterol from peripheral tissues including the arterial wall.37,38 Interestingly, excessive accumulation of cholesterol in tissues, such as that found in hypercholesterolemic Apoe−/− mice, leads to structural and functional defects of lymphatic vessels.39 In the same mouse model, restoration of lymphatic drainage improved cholesterol clearance.38 Thus, normal lymphatic function is essential for lipid clearance and could be a target for prevention or treatment of atherosclerotic vascular disease.
Skin lymphatic vessels have also been implicated in systemic blood pressure control through regulation of the electrolyte clearance.40 In turn, in hypertension, osmotic stress leads to inflammatory cell recruitment and increased lymphangiogenic growth factor production in the skin.40,41
In summary, lymphatic vasculature plays a key role in a number of physiological and pathological conditions. Studies of molecular mechanisms and signaling pathways that regulate development of lymphatic vasculature and the pathological lymphangiogenesis are actively underway. In this review, we describe the current view of several critical pathways regulating lymphangiogenesis with a major focus on the mechanisms in mammals (Figure 2). The latest view of lymphangiogenesis in zebrafish is presented in Impel and Schulte-Merker,42 whereas lymphatic vascular remodeling and the role of nonendothelial cells are reviewed in Schulte-Merker et al,1 Harvey and Gordon,20 and Alitalo.43
Signaling pathways in lymphatic vessels. Lymphatic sprouting and proliferation is regulated by a variety of external stimuli and intracellular signaling pathways. Loss of Vegf-c/Vegfr-3 or Ccbe1 completely prevents formation of lymphatic vessels, demonstrating a central role in lymphangiogenesis. Nrp2 and EphrinB2 enhance VEGF-C-dependent lymphangiogenic sprouting and signaling. Vegfr-2 also contributes to lymphangiogenic response. TGFβ/BMP and angiopoietin-2 are important both for lymphatic capillary patterning and maturation of collecting lymphatic vessels. Dll4/Notch signaling restricts LEC response to Vegf-a in adult tissues, whereas it has a prolymphangiogenic function during postnatal development. The Ras-RAF-mitogen-activated protein kinase (MEK)-extracellular signal-regulated kinase (ERK) pathway promotes lymphatic endothelial proliferation, likely downstream of VEGFR-3. Class I PI3 kinases are required for growth and remodeling of lymphatic vasculature in some vascular beds. Although strongly activated by (lymph)angiogenic growth factors in LECs in vitro, in vivo, the Ca2+/calcineurin pathway is mostly implicated in lymphatic collecting vessel development, in cooperation with Foxc2. Some pathways are also important for establishment of lymphatic endothelial cell identity, such as Notch and Raf/MEK/ERK1/2. Blue boxes indicate pathways also involved in sprouting. Foxc2 and calcineurin/Nfatc1 cooperatively regulate collecting vessel maturation (green box).
Signaling pathways in lymphatic vessels. Lymphatic sprouting and proliferation is regulated by a variety of external stimuli and intracellular signaling pathways. Loss of Vegf-c/Vegfr-3 or Ccbe1 completely prevents formation of lymphatic vessels, demonstrating a central role in lymphangiogenesis. Nrp2 and EphrinB2 enhance VEGF-C-dependent lymphangiogenic sprouting and signaling. Vegfr-2 also contributes to lymphangiogenic response. TGFβ/BMP and angiopoietin-2 are important both for lymphatic capillary patterning and maturation of collecting lymphatic vessels. Dll4/Notch signaling restricts LEC response to Vegf-a in adult tissues, whereas it has a prolymphangiogenic function during postnatal development. The Ras-RAF-mitogen-activated protein kinase (MEK)-extracellular signal-regulated kinase (ERK) pathway promotes lymphatic endothelial proliferation, likely downstream of VEGFR-3. Class I PI3 kinases are required for growth and remodeling of lymphatic vasculature in some vascular beds. Although strongly activated by (lymph)angiogenic growth factors in LECs in vitro, in vivo, the Ca2+/calcineurin pathway is mostly implicated in lymphatic collecting vessel development, in cooperation with Foxc2. Some pathways are also important for establishment of lymphatic endothelial cell identity, such as Notch and Raf/MEK/ERK1/2. Blue boxes indicate pathways also involved in sprouting. Foxc2 and calcineurin/Nfatc1 cooperatively regulate collecting vessel maturation (green box).
Cell surface receptors and their ligands in lymphangiogenesis
VEGF-C–VEGFR-3 signaling
VEGF-C, a member of the VEGF growth factor family, is a major regulator of formation and growth of lymphatic vessels, both in physiological and pathological contexts.1,43 In Vegfc−/− mice, LECs fail to migrate out of the veins to form primordial lymphatic vessels, and consequently, mice do not develop lymphatic vasculature.44 This function of Vegf-c is evolutionary conserved, as it is also required for the development of the zebrafish lymphatic vascular system.45 The lymphangiogenic activity of Vegf-c has been demonstrated in a range of animal models involving transgenic and knockout mice, viral gene delivery, and in in vitro assays, where VEGF-C promotes LEC proliferation, survival, and migration.25,46
Full-length VEGF-C binds receptor tyrosine kinase VEGFR-3 with high affinity, and further proteolytic processing generates a shorter isoform capable of also interacting with VEGFR-2.47 Vegfr-3 is initially expressed in the blood vasculature before becoming restricted to lymphatic vessels around midgestation. Vegfr-3 knockout mice die because of early blood vascular defects.48 Heterozygous missense mutations in Vegfr-3 have been linked to lymphedema in humans and in Chy mice.49,50 A loss-of-function VEGF-C mutation was also recently described in a family with the autosomal dominant form of lymphedema,51 and a truncation mutation in zebrafish Vegf-c severely affected lymphatic development.52 Vegf-c-dependent activation of Vegfr-3 is enhanced by mechanical stretch in an integrin β1-dependent manner, providing a mechanism of how increased interstitial pressure guides expansion of lymphatic vasculature.53
Neuronal guidance molecule neuropilin-2 (Nrp2) is highly expressed on lymphangiogenic vessels, where it acts as a Vegfr-3 coreceptor during Vegf-c-induced lymphatic sprouting. Nrp2−/− deficient mice have severe hypoplasia of lymphatic capillaries from E13 to birth.54 Furthermore, reduction of lymphatic vessel sprouting was observed in Nrp2+/−;Vegfr3+/− mice, but not in Nrp2+/−;Vegfr2+/− mice, providing genetic evidence for the role of Nrp2 in the Vegf-c/Vegfr-3 signaling cascade.55 Nrp2 levels are reduced in mice with endothelial-specific inactivation of COUP-TFII, and COUP-TFII acts as a direct regulator of Nrp2 expression in LECs.56 In addition, transcription factors GATA binding protein 1 (GATA1), GATA2, and LIM-domain-only protein (LMO)-2 regulate the expression Nrp2 on endothelial cells in vitro.57 Nrp2 is re-expressed during tumor lymphangiogenesis, and treatment with neutralizing anti-Nrp2 antibody decreases tumor lymphangiogenesis and metastasis to sentinel lymph node and distant organs.58
In addition to Vegfr-3, lymphatic vessels also express high levels of Vegfr-2, whereas Vegfr-1 is predominantly expressed by blood vessels.59 Inactivation of Vegfr-2 in LECs results in decreased lymphatic sprouting and proliferation, without affecting vessel caliber.60 Interestingly, the VEGFR-2-specific ligand, VEGF-E, induces circumferential growth without affecting lymphatic sprouting.61 Thus, it is possible that ligand-dependent Vegfr-2 signaling contributes to LEC proliferation, whereas sprouting is regulated through a ligand-independent Vegfr-2 modulation of Vegfr-3/Nrp2 signaling (eg, via Vegfr-2/3 heterodimers) present in the LEC filipodia.62
Collagen- and calcium-binding epidermal growth factor-like domains 1
Collagen- and calcium-binding epidermal growth factor-like domains 1 (Ccbe1) is a secreted extracellular matrix-binding protein, mutated in patients with Hennekam syndrome,63 a rare disease that presents with lymphedema, lymphangiectasia, and other pathological features.64 The function of Ccbe1 is evolutionary conserved, as it is required for embryonic lymphangiogenesis in zebrafish65 and mice.66 Ccbe1 is expressed in the somitic mesoderm during thoracic duct formation in zebrafish and in the areas adjacent to the anterior cardinal vein in mouse embryos, where it acts non–cell autonomously to regulate Vegf-c processing and bioavailability.65,67,68 Lymphangiogenesis is also potently induced on coadministration of Ccbe1 and Vegf-c in a corneal lymphangiogenesis mouse model.66 Ccbe1−/− mice develop severe anemia because of the defective definitive erythropoiesis, suggesting that Ccbe1 has additional,Vegf-c-independent function(s).69 Ccbe1 is a direct target of atypical E2F transcription factors E2F7/8. Importantly, inactivation of e2f7/8 in zebrafish reduced Ccbe1 expression and impaired venous sprouting and lymphangiogenesis, whereas overexpression of e2f7/8 rescued Ccbe1- and Vegfr-3-dependent lymphangiogenesis.70
Ephrin-Eph
Eph receptor tyrosine kinase receptors and their ligands, ephrins, are key regulators of axon guidance during nervous system development, and they also contribute to many aspects of blood vascular morphogenesis.71 In addition to acting as Eph-activating ligands (so-called forward signaling), ephrins are also able to initiate reverse signaling, which in the case of ephrinB ligands is achieved through the recruitment of adaptor proteins in the cytoplasmic portion. Lymphatic vessels express both EphrinB2 and EphB4, an ephrinB2 receptor.72,73 EphrinB2 promotes Vegf-c/Vegfr-3 signaling in the developing LECs through the regulation of Vegfr-3 internalization, important for full activation of downstream signaling pathways, such as Akt (also known as protein kinase B) and ERK.74 EphrinB2 has a similar function in the regulation of Vegfr-2 on blood vessels and platelet-derived growth factor receptor ß signaling in pericytes.75,76 Blocking ephrinB2 activity using antibodies prevents tumor angiogenesis and lymphangiogenesis, suggesting a potential therapeutic approach for targeting tumor neovascularization.77
Loss of the C-terminal PDZ (post synaptic density protein [PSD95], Drosophila disc large tumor suppressor [Dlg1], zonula occludens-1 protein [zo-1]) domain of ephrinB2, implicated in reverse signaling, also leads to defective remodeling of primary lymphatic plexus and failure to form collecting lymphatic vessels and lymphatic valves.72 Studies of adult corneal lymphatic vessels showed that EphB4 is highly expressed in the valves where administration of EphB4-Fc fusion proteins prevented regeneration of valves after corneal injury.73
Angiopoietins and Tie receptors
Tie1 and Tie2 endothelial receptor tyrosine kinases and their angiopoietin (Ang) ligands play important roles in blood vessel maturation, patterning, and stability, both in physiological and pathological conditions.78 The current view of Ang-Tie signaling in blood vessels suggests that Ang1 acts to stabilize vessels and limit angiogenesis,78 although this was challenged by a genetic study that found that Ang1 is dispensable for maintenance of quiescent vasculature in adult animals.79 Ang2 is in many cases antagonistic to Ang1, but it can be also agonistic under certain conditions, such as at high concentration and/or in tumor endothelial cells. Ang1, Tie1, and Tie2, but not Ang2, are essential for embryonic vascular development, and their inactivation leads to embryonic lethality at E12.5 (reviewed in Eklund and Saharinen78 ). Decreased dosage of Tie1 leads to embryonic edema and mispatterning of early lymphatic vasculature, without affecting the lymphatic endothelial commitment.80,81 It is not known whether these defects are a result of modified Ang1 or Tie2 signaling in LECs or whether Tie1 has an angiopoietin-independent role in lymphatic endothelium. In contrast to its limited role during physiological angiogenesis, Ang2 plays an essential role in lymphatic vasculature. Angpt2−/− mice have hypoplastic lymphatic capillaries and fail to form collecting vessels, which leads to lymphatic dysfunction and impaired postnatal survival.82,83 Interestingly, Ang1 rescues the lymphatic vascular, but not blood vascular defects in Angpt2−/− mice, and overexpression of Ang1 induces both physiological and pathological lymphangiogenesis in a Vegfr-3-dependent manner,82,84,85 highlighting the differences in the use of angiopoietin ligands by the 2 vascular systems. Studies of blood vasculature revealed important roles of the Ang-Tie pathway in a variety of pathological conditions, such as inflammation, fibrosis, and cancer, underlining their importance as therapeutic targets.78,86,87 However, the relative contribution of Ang-Tie signaling in lymphatic vessels to these processes still needs to be fully examined.
Notch signaling
Notch is a fundamental signaling pathway, which plays a key role in blood vascular development and function. The Notch pathway is mediated by Notch receptors (Notch1-Notch4), as well as Delta-like (Dll1, Dll3, Dll4) and Jagged (Jag1 and Jag2) ligands. Notch receptors are single-pass transmembrane proteins that function both as cell surface receptors and transcriptional regulators. Canonical Notch signaling is triggered via the interaction of Notch receptor and ligands on neighboring cells, which induces a conformational change in Notch receptors, cleavage of the Notch intracellular domain (NICD), and translocation of the NICD in the nucleus. The NICD interacts with DNA-binding protein RbpJ, constitutively recruited to the promoters of Notch target genes, and replaces the associated corepressor complex. Primary Notch target genes include Hes and Hey transcription factors.88 In blood vessels, Notch signaling regulates angiogenic sprouting, by controlling the selection of stalk and tip cells, the recruitment of SMC/pericytes, and arterio-venous differentiation.89
Cultured human LECs express high levels of DLL4, NOTCH1, NOTCH4, and JAG1.90-92 Blocking Notch signaling in cultured LECs and in adult mice using the Dll4-Fc fusion protein leads to lymphatic endothelial hypersprouting and increases responsiveness of LECs to Vegf-a, indicating that under these conditions Notch function is analogous to its role in blood vessels, ie, restriction of (lymph)angiogenic potential of endothelial cells.92 However, inactivation of Notch during early postnatal development or in a wound healing model in mice using Dll4 or Notch1 blocking antibodies or in zebrafish embryos using a morpholino oligo knockdown approach results in decreased lymphatic vessel sprouting.91,93
In vitro studies also suggested the role of NOTCH signaling in lymphatic endothelial differentiation through the repression of PROX1 and COUP-TFII.94 Down-regulation of Notch activity in vivo resulted in increased generation of LEC progenitors, whereas overexpression of NICD in LECs between E9.75 and E13.5 repressed COUP-TFII and Prox1,95 demonstrating that Notch acts as a negative regulator of LEC specification. Taken together, these data reveal a complex picture of Notch signaling in lymphatic vasculature, where it can be either pro- or antilymphangiogenic or regulate venous vs LEC fate decisions, probably depending on the developmental stage or tissues investigated.
Transforming growth factor/bone morphogenetic protein signaling
Transforming growth factor (TGF) and bone morphogenetic protein (BMP) signaling pathways induce pleiotropic tissue-specific responses by regulating proliferation, differentiation, migration, and cellular survival. TGF/BMP ligands (BMP2, BMP7, GDF5, BMP9, BMP10, AMH, TGF-β1/2/3, Activins, and Nodals) bind TGF receptors type II (TGFRII) (BMPR2, ACVR2A/B, and TGFβRII), which phosphorylate TGFRI (Alk1-7) via Ser/Thr tyrosine kinase activity. Downstream signaling pathways are transmitted to the cell nucleus via phosphorylation of Mad (mothers against decapentaplegic gene in Drosophila) and Sma genes (SMAD) transcription factors, which trigger target gene transcription.96 In addition, the noncanonical TGFβ signaling pathway regulates diverse cellular responses involving mitogen-activated protein kinases (MAPK), ρ-like GTPases, and PI3k/Akt.97 Endothelial cells signal through 2 type I TGFRs: Alk5 (TGFβRI) and Alk1 (ACVRL1), which activate SMAD2/3 and SMAD1/5, respectively.97 Mutations of the components of these signaling pathways cause several human hereditary vascular diseases, such as hereditary hemorrhagic telangiectasia (ENDOGLIN or ALK1) or pulmonary arterial hypertension (BMPR2).97 Studies of genetic mouse models revealed critical roles of TGF-β/BMP signaling in angiogenic sprouting.98-100 TGF/BMP also play an important role in the regulation of vascular integrity in the brain,101 maintenance of the quiescent vasculature,102 and SMC differentiation and recruitment to blood endothelial cells.103-105
Cultured LECs express Alk1, Alk2, Alk4, Alk5, ACVR2B, BMPR2, Endoglin, and TGFβRII receptors.106 In vitro treatment of LECs with TGFβ-1 reduces cell proliferation, cord formation, and expression of lymphatic markers Prox1 and lymphatic vessel endothelial hyaluronic acid receptor 1 (Lyve-1).107 TGFβ-1 inhibits lymphatic endothelial commitment and down-regulates related markers, such as COUP-TFII and Sox18, in murine embryonic stem cell-derived Flk1+ cardiovascular progenitors,107 although in another study, TGFβ-2 treatment increased expression of Vegfr-3 and Nrp2, both involved in lymphatic sprouting events rather that lymphatic endothelial commitment.108 Similarly, BMP9 suppresses the expression of LYVE1 and PROX1.109,110
In contrast to the in vitro results, endothelial-specific loss of TgfbrI (Alk5) or TgfbrII during embryogenesis did not affect lymphatic endothelial cell commitment. Instead, it impaired formation of tip cells and reduced complexity of skin lymphatic network, leading to hyperplasia of cutaneous lymphatic vessels.108 These in vivo results suggest that TGFβ signaling is an important regulator of lymphatic network patterning.108 BMP2 is known to interact with BMPR2, ACVR2A, or ACVR2B as type II TGF receptors and Alk-2, Alk-3, or Alk-4 as type I receptors. In zebrafish embryos, Bmp2 signaling pathway acts as a negative regulator of LEC emergence from veins, and overexpression of Bmp2b suppresses formation of lymphatic vascular structures, presumably due to the loss of Prox1a.111 However, it should be noted that the genetic inactivation of Prox1a does not affect zebrafish lymphangiogenesis.11
Inhibition of TGFβ/BMP signaling during the early postnatal development using Alk1, Acvr2b, and Bmbpr2 blocking antibodies prevented growth of lymphatic vessels in several organs, including the skin and intestine, without affecting collecting lymphatic vessels.106 This effect was further enhanced by blocking Vegfr-3 signaling. Thus, Alk1 signaling appears to play a major role in postnatal lymphangiogenesis, and in its absence, lymphatic vessels become more sensitive to Vegfr-3–Vegf-c/d depletion.106 At present, it is not clear what ligand is responsible for this effect. Indeed, targeted inactivation of high-affinity Alk1 ligand Bmp9 has an opposite effect, as it induces lymphatic vascular hyperplasia and lymphatic valve defects109,110 ; thus, additional Alk1 ligands that may be contributing to lymphangiogenesis remain to be investigated.
Several studies also addressed the question of the role of TGFβ signaling in pathological lymphangiogenesis. Treatment using a small-molecule TGFβRI inhibitor increased lymphangiogenesis in chronic peritonitis and tumor xenografts.107 In a model of tail lymphedema, treatment with blocking TGFβ antibody similarly increased lymphangiogenesis and alleviated inflammation, lymphedema, and fibrosis.112
To conclude, TGF/BMP signaling is important for several steps of lymphatic vascular development, including sprouting/tip cell formation, regulation of cell proliferation, and formation of specialized lymphatic structures, such as intraluminal valves.107,108,113 The future challenge will be to elucidate the precise roles of different receptors and ligands in these processes, as well as to understand potential combinatorial interactions with other signaling pathways, such as Notch, already revealed in blood vasculature.99,100
Intracellular signaling pathways in lymphangiogenesis
Lymphangiogenesis is regulated by a number of ligand-receptor interactions, which in turn activate intracellular signaling pathways responsible for transmitting information within the cell, and ultimately endothelial cell responses, such as proliferation, survival, or migration. Much effort is dedicated to studies of such ligands and receptors, because they represent important targets for drug development. However, in the last few years, novel knowledge has also been accumulated on the intracellular pathways, which in many cases revealed surprising differences between the blood and lymphatic vasculature (Table 1114-128 ). In the following sections, we will describe the current knowledge on the major intracellular signaling pathways in lymphangiogenesis.
Comparison of blood and lymphatic vascular phenotypes in loss-of-function genetic mouse models
| Genes . | Blood vessel phenotype . | Lymphatic vessel phenotype . |
|---|---|---|
| Ligands and receptors | ||
| Vegf-c | Vegfc−/−: Normal developmental angiogenesis44 | Vegfc−/−: No sprouting of LECs, absence of lymphatic vessels44 Vegfc+/−: Chylous ascites, hypoplasia44 |
| Vegfr-3 | Vegfr3−/−: Early blood vessel remodeling defects, death at E9.5-1048 Vegfr3f/f;Pdgfb-CreERT2: Vessel hypersprouting114 | Vegfr3+/Chy: Chylous ascites, hypoplasia50 Vegfr3ΔLBD/ΔLBD: No sprouting of LECs due to the removal of ligand-binding (LBD) domain of Vegfr-3115 |
| Nrp2 | Nrp2−/−: Normal developmental angiogenesis54 | Nrp2−/−: Hypoplasia of capillaries E13 to birth54 Nrp2+/−;Vegfr3+/−: Decreased vessel sprouting55 |
| EphrinB2 | Efnb2f/f;Cdh5-CreERT2: Reduced vascular network complexity, sprouting and endothelial cell proliferation74 Efnb2ΔV/ΔV: Normal developmental angiogenesis72 | Efnb2f/f;Cdh5-CreERT2: Decreased vessel sprouting74 Efnb2ΔV/ΔV: Defective lymphatic vascular remodeling, hyperplasia, valve agenesis72,74 |
| Vegfr-2 | Vegfr2−/−: Lack of development of the blood islands and embryonic vasculature, death at E8.5-E9.5116 Vegfrf/f;Lyve-1Cre: Decreased blood vessel density in the yolk sac, liver and lung60 | Vegfrf/f;Lyve-1Cre: Hypoplasia, decreased vessel sprouting60 |
| Bmp9 | Bmp9−/−: No phenotype in the retinal blood vessels117 | Bmp9−/−: Postnatal vessel hyperplasia, decreased maturation of valves. Adults: enlarged lymphatics, decreased number of lymphatic valves, impaired lymph drainage109,110 |
| TGFβ2 | Tgfb2−/−: Blood vascular remodeling occurs normally108 | Tgfb2−/−: Mild edema, increased cellular proliferation, decreased complexity and sprouting, enlarged vessels108 |
| TGFβRI | TgfbrIf/f;Tie1Cre: Defective vascular network, pericardial effusion in the heart, abnormalities in the vasculature of the yolk sac at E9.5, lethal at E10.5103 TgfbrIf/f;Tie2Cre: Hypoplastic endocardial cushion in the arterio-ventricular canal; thinner, poorly trabeculated myocardium, lethal soon after E13118 TgfbrIf/f;Alk1GFPCre/+: Enlarged pericardial cavity, underdeveloped heart, lethal at E14.5102 | TgfbrIf/fProx1+/GFPCre: Deletion at E9.5-10.5. Edema at E14.5 with blood filled lymphatics. Lymphatic network and sprouting reduction106 TgfbrIf/f;Prox1-CreERT2: Deletion at E12.5 impaired cell sprouting and hyperplasia106 TgfbrIf/f;VEC-CreERT2: Deletion at E12.5, mild edema, hyperplasia, lymphatic network and sprouting reduction106 |
| TGFβRII | TgfbrIIf/f;Tie1Cre: Pericardial effusion in the heart, abnormalities in the vasculature of the yolk sac at E9.5, lethal at around E10.5103 TgfbrIIf/fl;Tie2Cre: Cardiac defects and lethality at around E12.5119 TgfbrIIf/f;Pdgfb-CreERT2: Hemorrhagic blood vessels in retina, impaired vascular development96 | TgfbrIIf/f;Prox1+/GFPCre: Deletion at E9.5-10.5. Edema at E14.5 with blood – filled lymphatics. Lymphatic network and sprouting reduction106 TgfbrIIf/f;Prox1-CreERT2: Deletion at E12.5 presence of dysmorphogenic lymphatic vessels and reduced lymphatic branching106 TgfbrIIf/f;VEC-CreERT2: Deletion at E12.5, mild edema, hyperplasia, lymphatic network and sprouting reduction106 |
| Notch1 | Notch1f/f;Cdh5-CreERT2: Regional retinal vessel hypersprouting120 | Notch1f/f;Prox1CreERT2: Overproduction of LECs, edema, blood-filled lymphatics and incorporation of BECs into the peripheral lymphatics95 |
| Ccbe1 | Ccbe1−/−: Normal physiological angiogenesis66 | Ccbe1−/−: No sprouting of LECs, absence of lymphatic vessels66 Ccbe1+/−: Early irregular formation of intersegmental veins; no lymphatic phenotype later7 |
| Angipoietins/Tie | Angpt1−/−, Tie1−/−, Tie2−/−: Defective embryonic blood vessel remodeling, lethality at E12.548121 Angpt2−/−: Normal embryonic angiogenesis, defective hyaloid vessel regression82 | Angpt2−/−: Hypoplasia of lymphatic capillaries, failure to form collecting vessels82,83 Tie1−/−: Abnormal patterning, dilated and disorganized lymphatic vessels80,81 |
| Intracellular pathways | ||
| H/N/Kras | Nras+/−;Kras+/−: Normal developmental angiogenesis122 | Nras+/−;Kras+/−: Lymphatic hypoplasia, chylous ascites122 |
| Rasa1 | Rasa1−/−: Early blood vascular remodeling defects, lethality at E9.5123 Rasa1f/f;UB-ERT2Cre: Systemic inactivation in adults-normal blood vessels124 | Rasa1f/f;UB-ERT2Cre: Systemic inactivation in adults leading to hyperplasia, increased leakage, chylothorax, chylous ascites124 |
| PI3K/Akt1 | Pi3kca−/−(p85/p55/p50): Minor defects of developmental angiogenesis125 Akt1−/−: Normal physiological angiogenesis126 | Pi3kca−/−(p85/p55/p50): Impaired valve development, organ-specific hypoplasia125 Pi3kp110Rbd/Rbd: Chylous ascites, decreased branching and network complexity125 Akt1−/−: Reduced capillary density, defective valve development140 |
| Cnb1 | Cnb1f/f;Tie2-Cre: Defective coronary vasculature127 Cnb1f/f;Pdgfb-CreERT2: Normal physiological angiogenesis128 | Cnb1f/f;Prox1-CreERT2: Defective lymphatic vessel maturation and valve development128 |
| Genes . | Blood vessel phenotype . | Lymphatic vessel phenotype . |
|---|---|---|
| Ligands and receptors | ||
| Vegf-c | Vegfc−/−: Normal developmental angiogenesis44 | Vegfc−/−: No sprouting of LECs, absence of lymphatic vessels44 Vegfc+/−: Chylous ascites, hypoplasia44 |
| Vegfr-3 | Vegfr3−/−: Early blood vessel remodeling defects, death at E9.5-1048 Vegfr3f/f;Pdgfb-CreERT2: Vessel hypersprouting114 | Vegfr3+/Chy: Chylous ascites, hypoplasia50 Vegfr3ΔLBD/ΔLBD: No sprouting of LECs due to the removal of ligand-binding (LBD) domain of Vegfr-3115 |
| Nrp2 | Nrp2−/−: Normal developmental angiogenesis54 | Nrp2−/−: Hypoplasia of capillaries E13 to birth54 Nrp2+/−;Vegfr3+/−: Decreased vessel sprouting55 |
| EphrinB2 | Efnb2f/f;Cdh5-CreERT2: Reduced vascular network complexity, sprouting and endothelial cell proliferation74 Efnb2ΔV/ΔV: Normal developmental angiogenesis72 | Efnb2f/f;Cdh5-CreERT2: Decreased vessel sprouting74 Efnb2ΔV/ΔV: Defective lymphatic vascular remodeling, hyperplasia, valve agenesis72,74 |
| Vegfr-2 | Vegfr2−/−: Lack of development of the blood islands and embryonic vasculature, death at E8.5-E9.5116 Vegfrf/f;Lyve-1Cre: Decreased blood vessel density in the yolk sac, liver and lung60 | Vegfrf/f;Lyve-1Cre: Hypoplasia, decreased vessel sprouting60 |
| Bmp9 | Bmp9−/−: No phenotype in the retinal blood vessels117 | Bmp9−/−: Postnatal vessel hyperplasia, decreased maturation of valves. Adults: enlarged lymphatics, decreased number of lymphatic valves, impaired lymph drainage109,110 |
| TGFβ2 | Tgfb2−/−: Blood vascular remodeling occurs normally108 | Tgfb2−/−: Mild edema, increased cellular proliferation, decreased complexity and sprouting, enlarged vessels108 |
| TGFβRI | TgfbrIf/f;Tie1Cre: Defective vascular network, pericardial effusion in the heart, abnormalities in the vasculature of the yolk sac at E9.5, lethal at E10.5103 TgfbrIf/f;Tie2Cre: Hypoplastic endocardial cushion in the arterio-ventricular canal; thinner, poorly trabeculated myocardium, lethal soon after E13118 TgfbrIf/f;Alk1GFPCre/+: Enlarged pericardial cavity, underdeveloped heart, lethal at E14.5102 | TgfbrIf/fProx1+/GFPCre: Deletion at E9.5-10.5. Edema at E14.5 with blood filled lymphatics. Lymphatic network and sprouting reduction106 TgfbrIf/f;Prox1-CreERT2: Deletion at E12.5 impaired cell sprouting and hyperplasia106 TgfbrIf/f;VEC-CreERT2: Deletion at E12.5, mild edema, hyperplasia, lymphatic network and sprouting reduction106 |
| TGFβRII | TgfbrIIf/f;Tie1Cre: Pericardial effusion in the heart, abnormalities in the vasculature of the yolk sac at E9.5, lethal at around E10.5103 TgfbrIIf/fl;Tie2Cre: Cardiac defects and lethality at around E12.5119 TgfbrIIf/f;Pdgfb-CreERT2: Hemorrhagic blood vessels in retina, impaired vascular development96 | TgfbrIIf/f;Prox1+/GFPCre: Deletion at E9.5-10.5. Edema at E14.5 with blood – filled lymphatics. Lymphatic network and sprouting reduction106 TgfbrIIf/f;Prox1-CreERT2: Deletion at E12.5 presence of dysmorphogenic lymphatic vessels and reduced lymphatic branching106 TgfbrIIf/f;VEC-CreERT2: Deletion at E12.5, mild edema, hyperplasia, lymphatic network and sprouting reduction106 |
| Notch1 | Notch1f/f;Cdh5-CreERT2: Regional retinal vessel hypersprouting120 | Notch1f/f;Prox1CreERT2: Overproduction of LECs, edema, blood-filled lymphatics and incorporation of BECs into the peripheral lymphatics95 |
| Ccbe1 | Ccbe1−/−: Normal physiological angiogenesis66 | Ccbe1−/−: No sprouting of LECs, absence of lymphatic vessels66 Ccbe1+/−: Early irregular formation of intersegmental veins; no lymphatic phenotype later7 |
| Angipoietins/Tie | Angpt1−/−, Tie1−/−, Tie2−/−: Defective embryonic blood vessel remodeling, lethality at E12.548121 Angpt2−/−: Normal embryonic angiogenesis, defective hyaloid vessel regression82 | Angpt2−/−: Hypoplasia of lymphatic capillaries, failure to form collecting vessels82,83 Tie1−/−: Abnormal patterning, dilated and disorganized lymphatic vessels80,81 |
| Intracellular pathways | ||
| H/N/Kras | Nras+/−;Kras+/−: Normal developmental angiogenesis122 | Nras+/−;Kras+/−: Lymphatic hypoplasia, chylous ascites122 |
| Rasa1 | Rasa1−/−: Early blood vascular remodeling defects, lethality at E9.5123 Rasa1f/f;UB-ERT2Cre: Systemic inactivation in adults-normal blood vessels124 | Rasa1f/f;UB-ERT2Cre: Systemic inactivation in adults leading to hyperplasia, increased leakage, chylothorax, chylous ascites124 |
| PI3K/Akt1 | Pi3kca−/−(p85/p55/p50): Minor defects of developmental angiogenesis125 Akt1−/−: Normal physiological angiogenesis126 | Pi3kca−/−(p85/p55/p50): Impaired valve development, organ-specific hypoplasia125 Pi3kp110Rbd/Rbd: Chylous ascites, decreased branching and network complexity125 Akt1−/−: Reduced capillary density, defective valve development140 |
| Cnb1 | Cnb1f/f;Tie2-Cre: Defective coronary vasculature127 Cnb1f/f;Pdgfb-CreERT2: Normal physiological angiogenesis128 | Cnb1f/f;Prox1-CreERT2: Defective lymphatic vessel maturation and valve development128 |
RBD, Ras-binding domain.
Ras/Raf/MEK/ERK
The Ras family of GTPases (Hras, Nras, and Kras) plays essential roles in a variety of cellular functions, such as proliferation, migration, differentiation, and apoptosis, frequently downstream of receptor tyrosine kinases. Ras is a small molecule GTPase, which in its active, GTP-bound state, can activate multiple downstream effectors, including the Raf/MEK/ERK pathway and class I phosphatidylinositol 3 kinases (PI3Ks). A variety of associated proteins (guanine-nucleotide exchange factors) increase Ras GTPase activity by promoting GDP-GTP exchange, whereas GTPase-activating proteins, or Ras-GAPs, stimulate the hydrolysis of GTP on Ras and are important for Ras inactivation.129 In the Raf/MEK/ERK pathway, active Ras interacts with RAF1 Ser/Thr kinase, which in turn activates downstream MEK kinases. MEK kinases then phosphorylate and activate ERK1/2 MAPK.130
Ras signaling plays an important role in lymphatic vessel development and function, likely downstream of the VEGFR-3 pathway. Nras+/−Kras+/− mice display lymphatic hypoplasia and chylous ascites, which are rescued by overexpression of Hras.122 Conversely, mice that overexpress Hras in endothelial cells have lymphatic vessel hyperplasia, driven by increased levels of Vegfr-3 and enhanced MAPK signaling. However, such hyperplastic vessels are not fully functional, as transgenic mice develop edema and chylothorax. In humans, chylous ascites, chylothorax and lymphedema are associated with activating mutations in KRAS in cardiofaciocutaneous syndrome131 and HRAS in Costello syndrome.132,133 The work in mouse models also revealed differential sensitivity of blood vs lymphatic vessels to altered Ras signaling, as blood vessel development or function was not affected on Hras overexpression or in Kras+/−, Nras+/− mice.122
Negative regulator of Ras activity p120-RasGap (RASA1) is mutated in human hereditary disease capillary malformation–arteriovenous malformation (CM-AVM), characterized by multiple cutaneous vascular lesions and high risk for development of fast-flow blood vascular lesions.134 Lymphatic malformations were observed in some CM-AVM patients,135 and recent advanced imaging analysis revealed hyperplastic cutaneous lymphatic vessels in a CM-AVM patient, suggesting that lymphatic vascular dysfunction may be frequent in CM-AVM.136 Inactivation of Rasa1 in adult mice causes extensive Vegfr-3-dependent lymphatic vessel hyperplasia and lymphatic vessel leakage, leading to chylothorax, chylous ascites, and animal death.124 Importantly, no blood vasculature phenotype was observed on loss of Rasa1 in adult mice, unlike in Rasa1-deficient embryos,123 implying that blood vessels need Ras regulation only during embryonic angiogenesis, whereas lymphatic vessels require a lifelong suppression of Ras signaling. A number of other Ras signaling effectors and regulators have been implicated in vascular development and angiogenesis, such as Rasip1137 ; therefore, it will be interesting to investigate and compare their function in lymphatic vessels as well.
Studies of blood vasculature revealed important interactions of PI3K-Akt and Erk signaling in the regulation of arterio-venous differentiation, in which high PI3K-Akt signaling favors venous fate and vein formation, whereas arterial morphogenesis is induced by Erk1/2 MAPK under conditions of attenuated PI3K-Akt activation.138,139 In addition to the regulation of lymphatic hyperplasia downstream of VEGFR-3 signaling, as discussed above, MAPK/ERK cascade might be also important for the establishment of LEC fate. Endothelial-specific expression of activated MAPK via upstream regulator Raf1, increased commitment of venous endothelial cells to the lymphatic fate and even induced lymphatic markers in arterial endothelial cells through the induction of Sox18 and Prox1.140 Spatially localized, yet unknown, signal may therefore regulate Erk activation in embryonic veins and lead to the establishment of lymphatic endothelial fate in a subpopulation of venous ECs.
Class I PI3Ks
PI3Ks are a family of enzymes that phosphorylate the 3-OH group of inositol membrane lipids and produce a variety of 3-phosphorylated phosphoinositides. The latter propagate intracellular signaling by providing docking sites for pleckstrin-homology domains of diverse signaling proteins. Downstream effectors of PI3K include the serine/threonine kinase Akt that, among other pathways, activates mammalian target of rapamycin (mTOR) signaling, which is central for cellular proliferation, survival, and metabolism. The PI3K/Akt/mTOR pathway is frequently activated in cancers, and a number of PI3K and mTOR inhibitors are either in clinical trials or already approved for treatment.141,142
Based on their primary structure, regulation, and substrate specificity, 3 classes of PI3Ks (Ia, Ib, II, and III) were identified. Class Ia PI3K is a group of dimeric proteins containing a catalytic subunit (p110α, p110β, or p110δ) and a regulatory subunit (p85α, p55α, p50α, p85β, or p55γ) that are activated by a variety of receptor tyrosine kinases. Regulatory p85 subunit directly binds phosphotyrosines on receptor tyrosine kinases or adaptor proteins, which leads to PI3K activation. PI3K can also be activated directly by Ras, which interacts with the p110 subunit.143 Studies of animal models have uncovered multiple functions of class I PI3Ks in blood vascular development, such as regulation of blood vessel integrity, angiogenic sprouting, and remodeling, as well as arterio-venous differentiation.144 VEGF-A, VEGF-C, and VEGF-D activate the PI3K-Akt signaling cascade in cultured LECs, where this pathway is important for migration145 via a direct interaction with VEGFR-3.146 LECs express p110α, -β, and -δ but not -γ isoforms, where p110α is a major isoform, responsible for VEGF-C-dependent Akt activation and LEC migration.147 Germ-line deletion of Pik3r1, encoding the regulatory subunits p85α, p55α, and p50α of class I PI3Ks, led to chylous ascites, suggesting lymphatic vascular dysfunction.148 Further analysis of these mice revealed organ-specific defects in postnatal lymphatic sprouting and lymphatic maturation, without major impact on blood vessel development.125 Mutations in the Pi3kca encoding the catalytic p110α isoform, which block its interaction with Ras, led to lymphatic vessel hypoplasia, reduced sprouting, and perinatal chylous ascites.149 In a tumor setting, p110α signaling downstream of VEGF-C is important for the activation of integrin α4β1 on lymph node lymphatic endothelium and subsequent adhesion of metastatic tumor cells.147 In human disease, somatic activating mutations in PI3KCA were found in patients with Congenital Lipomatous Overgrowth, Vascular Malformations and Epidermal Nevi Syndrome and Klippel-Trenaunay-Weber syndrome, which present with malformation or overgrowth of lymphatic vessels in addition to tissue overgrowth and other vascular anomalies.150
Three highly related isoforms of Akt serine/threonine kinase (Akt1, Akt2, and Akt3) represent the major signaling arm of PI3K. All isoforms are expressed in LECs; however, Akt1 has a dominant role in lymphatic vasculature, as Akt1−/−, but not Akt2−/− or Akt3−/−, mice display reduced capillary density and defective valve development.126 Interestingly, VEGF-C-induced lymphangiogenesis is normal in Akt1−/− mice, indicating either compensation by other isoforms or a predominant role in cell survival rather than migration or proliferation.126 As observed in mice with mutant Ras or PI3K signaling components, the physiological angiogenesis was not affected in the absence of Akt1. The activating AKT1 mutation underlies Proteus syndrome, characterized by general tissue overgrowth, as well as cutaneous vascular lymphatic or lympho-venous malformation.151
Ca2+/calcineurin/NFAT cells signaling
Calcineurin is a calcium-activated protein phosphatase, which plays a major role in calcium signaling in different cells types, and it is a target of immunosuppressive drugs cyclosporine A and tacrolimus. An increase in intracellular calcium activates calcineurin and leads to dephosphorylation and nuclear translocation of the transcription factors of the nuclear factor of activated T cells (NFAT) family, which act as main effectors of the Ca2+/calcineurin pathway. In vitro studies suggested that calcineurin/NFAT mediates most VEGF/VEGFR-2-induced responses of endothelial cells, including cell proliferation, migration, and tube formation.152-154 However, mice with constitutive endothelium-specific inactivation of calcineurin display coronary vessel-specific, but not generalized, angiogenesis defects.127 Angiogenesis proceeds normally on inducible inactivation of calcineurin in blood vasculature after E13.5, when the requirements for calcineurin signaling in heart development are bypassed.128 These data suggest a limited vascular bed-specific role for endothelial calcineurin signaling during physiological angiogenesis. Calcineurin may be more important for pathological angiogenesis, as mice deficient in Down syndrome critical region gene 1, an endogenous inhibitor of calcineurin, have decreased tumor angiogenesis.155
To date, of the 5 members of the NFAT family, only NFATc1 (NFAT2) is known to be implicated in lymphatic vascular development.156,157 In LECs, expression of NFATc1 is controlled by PROX1, whereas its nuclear translocation can be induced by VEGF-C through the activation of VEGFR-2 or by flow shear stress. The Ca2+/calcineurin/NFAT pathway cooperates with FOXC2, a major regulator of collecting vessel phenotype.128,156 Pharmacological inhibition or lymphatic-endothelial-specific inactivation of calcineurin prevents maturation of lymphatic vessels and arrests the development of lymphatic valves.128,156 Thus, similar to blood vasculature, the role of calcineurin signaling in lymphatic vessels in physiological conditions is restricted to a specific vascular compartment.
MicroRNAs and lymphangiogenesis
MicroRNAs (miRs) are short noncoding single stranded RNAs, which act as post-transcriptional regulators by inhibiting mRNA translation and/or promoting mRNA degradation. The same miR can regulate many mRNAs; therefore, miRs act as broad regulators of gene networks, akin to the transcription factors, although miRs act not as binary on and off switches, but rather to fine-tune the genetic program. The number of known miRs that regulate angiogenesis either in a cell autonomous manner or through the regulation of angiogenic factors in other cells is constantly increasing.158-160 However, in contrast to the wealth of information on miRs in angiogenesis, little is known about specific miR functions in LECs. miR-181a is expressed at higher levels in blood endothelial cells (BECs) in comparison with LECs, and it targets Prox1 mRNA for degradation. Increasing miR-181a levels in LECs reduced Prox1 expression and shifted LECs toward a blood vascular phenotype, suggesting that miR-181a acts to maintain the blood vascular phenotype.161 In another study, quantitative reverse transcription-polymerase chain reaction profiling of 157 miRs in cultured LECs and BECs identified LEC-specific miR-95 and miR-326 and BEC-specific miR-137, miR-31, miR-125b, and miR-99a.162 Further analysis demonstrated that miR-31 suppresses a number of lymphatic endothelial-specific genes, and this effect is at least partially due to the direct suppression of PROX1.162 Thus, higher expression of miR-31 and miR-181a may be important for preventing the acquisition of LEC identity by BECs. Factors regulating the expression of miR-31 and miR-181a in endothelial cells include BMP2.111
Concluding remarks
Lymphatic vessels perform a vast array of functions both in physiological and pathological conditions. Understanding molecular mechanisms regulating lymphatic vascular growth and remodeling should result in the design of new therapies for many human diseases. In the last few years, in addition to the well-established roles of VEGF-C/VEGFR-3 signaling in lymphangiogenesis, important contributions were uncovered for the Notch, TGFβ/BMP, Ras, MAPK/ERK, PI3K/Akt, and Ca2+/calcineurin pathways. It is noteworthy that in many cases lymphatic and blood vasculature demonstrates differential responses or use of these signaling pathways (Table 1). One of the future challenges will be to decipher the complexity of such signaling in different lymphatic vascular beds and disease conditions, and to identify critical determinants, which could be used for modulation of lymphatic function.
Acknowledgments
The authors apologize for not being able to cite all original research contributions due to space limitations. The authors thank Dr Jeremiah Bernier-Latmani and Dr Amélie Sabine for critical reading of the manuscript.
The research in T. V. Petrova’s laboratory is supported by Swiss National Science Foundation grants PPP0033-114898 and CRSII3-141811, Oncosuisse grant 2863-08-2011, the Leenaards Foundation, the Gebert Rüf Foundation, and the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme FP7/2007-2013/ under REA grant agreement 317250.
Authorship
Contribution: S.C., E.B., and T.V.P. conceived and wrote the manuscript and gave final approval for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tatiana V. Petrova, Department of Oncology, CHUV-UNIL, Ch. des Boveresses 155, CH-1066 Epalinges, Switzerland; e-mail: tatiana.petrova@unil.ch.