In this issue of Blood, Taylor et al uncovered a novel role for serum response factor (SRF) in neutrophil migration to inflamed tissues. Unexpectedly, SRF controls neutrophil migration via CD11b integrin clustering and trafficking.1
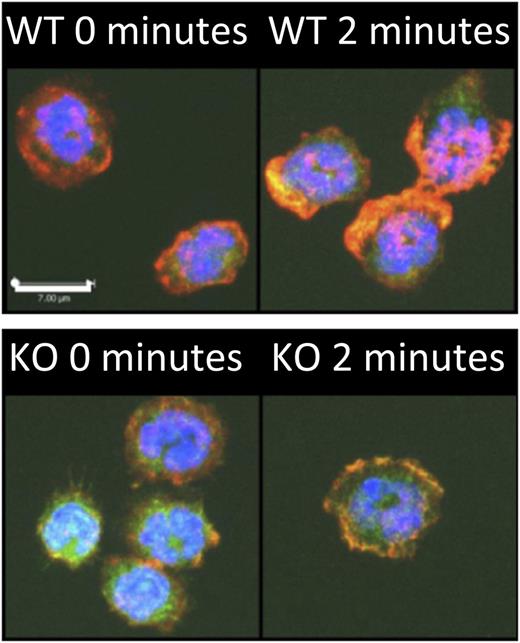
Srf is critical for neutrophil actin assembly. Srf wild-type (WT) and knockout (KO) neutrophils were allowed to attach to poly-l-lysine–coated coverslips and stimulated with N-formyl-methionyl-leucyl-phenylalanine for 2 minutes. F-actin was stained with phalloidin (red) in Cre-expressing neutrophils marked by yellow fluorescent protein; a merge with 4,6 diamidino-2-phenylindole is shown. Note the lack of polarization with decreased F-actin polymerization in Srf-null neutrophils compared with WT cells when activated with N-formyl-methionyl-leucyl-phenylalanine. See Figure 4A in the article by Taylor et al that begins on page 3027.
Srf is critical for neutrophil actin assembly. Srf wild-type (WT) and knockout (KO) neutrophils were allowed to attach to poly-l-lysine–coated coverslips and stimulated with N-formyl-methionyl-leucyl-phenylalanine for 2 minutes. F-actin was stained with phalloidin (red) in Cre-expressing neutrophils marked by yellow fluorescent protein; a merge with 4,6 diamidino-2-phenylindole is shown. Note the lack of polarization with decreased F-actin polymerization in Srf-null neutrophils compared with WT cells when activated with N-formyl-methionyl-leucyl-phenylalanine. See Figure 4A in the article by Taylor et al that begins on page 3027.
Neutrophils are the first line of host defense against invading pathogens because they migrate rapidly to the site of tissue infection.2 A tight regulation of neutrophil accumulation into tissues is critical for maintaining appropriate innate immunity and inflammatory responses. Neutrophil migration requires actin filament assembly to form the lamellipodium in the direction of migration. This is coordinated with cell adhesion via integrin receptors to anchor the cell to the extracellular matrix, enabling displacement of the cell body. The rear of the cell is enriched in an actomyosin network that provides the contractile force to allow cell detachment and propulsion.3 Actin assembly, which involves the polymerization of a single molecule of G-actin into filamentous actin, is essential for each of these steps to proceed.
SRF is a master regulator of the actin cytoskeleton assembly4 and a highly conserved transcription factor that is widely expressed. It controls the expression of genes encoding actin cytoskeleton and contractile proteins. Underscoring its importance is the observation of a defective embryo development at gastrulation of SRF-null mice. Yet, this widely expressed transcription factor can direct distinct gene expression programs by recruiting a variety of transcriptional cofactors. As such, the use of select Cre-expressing mice revealed that SRF functions—and the underlying mechanism SRF controls—are highly context- and cell type–specific. In the hematopoietic system, SRF is notably essential for megakaryopoiesis and platelet functions5 and hematopoietic stem cell integrin expression and functions.6 Noteworthy for this commentary is that myelodysplastic syndromes (MDS) are frequently associated with increased risk of infections. Genetic deletion in chromosome 5, which contains several genes of the “SRF pathway,” are common in MDS.7 Despite its importance, the role of SRF has not been studied in neutrophils.
In their study, Taylor et al1 used Srf-fl/fl mice crossed with doxycycline-inducible, Cre-expressing mice and performed bone marrow transplantation to delete Srf alleles in adult hematopoietic cells. This study demonstrates that SRF expression is absolutely critical for recruiting neutrophils to inflamed lungs in response to lipopolysaccharide (LPS) challenge. In the absence of SRF, neutrophils are unable to emigrate into the LPS-challenged lungs because of a neutrophil cell–intrinsic defect in migration toward a chemotactic gradient, as seen in vitro and in vivo. Cell adhesion is also decreased. Also noteworthy is that the chemokine-induced actin assembly into a broad lamellipodium normally located at the cell front is absent in SRF-null neutrophils (see figure). Instead, SRF-null neutrophils exhibit a modest and nonpolarized actin network. Hence, this study clearly demonstrates the critical role of SRF in neutrophils.
Quite interesting and of considerable importance is the mechanism by which SRF controls migration and actin responses in neutrophils. The authors discovered that SRF controls integrin affinity conformation and what may be a clathrin-dependent integrin-trafficking mechanism. Indeed, neutrophils express adhesion receptors of the β2 integrin family, including LFA-1 (CD11a/CD18) and MAC-1 (CD11b/CD18), which bind to intercellular adhesion molecule-1 (ICAM-1) on the endothelial cell surface. Integrin functions are highly regulated and involve activation of intracellular regulatory proteins and signaling pathway (inside-out signaling) to open their extracellular conformation, enabling ligand binding. Integrins can also redistribute and form intramembrane clusters that reinforce cell adhesion.8 Integrins are not only adhesion receptors but also play significant roles in maintaining neutrophil actin assembly and polarity during directional movement. The latter requires a coordinated regulation of integrin trafficking via clathrin-dependent endocytosis and recycling.9 In their study, Taylor et al show that in contrast to other hematopoietic cells, the expression of CD11b is consistently higher in SRF-null neutrophils, but the ability to bind to ICAM-1 is drastically decreased. Moreover, CD11b fails to adopt a polarized distribution and to colocalize with clathrin-enriched membrane domains when SRF is absent. A likely result of this would be a lack of CD11b internalization and recycling. Hence, SRF, either directly via actin reorganization or indirectly via expression of other target genes, represents an important regulator of CD11b trafficking from the front to the back of the cells—a critical step for maintaining cell shape integrity and directional movement. Although the authors did not completely examine how SRF may control clathrin-mediated CD11b trafficking, gene expression analysis using RNA deep sequencing may provide some insights because it could reveal a novel SRF gene expression signature in neutrophils. Most notably, in addition to the expected actin cytoskeletal genes, SRF also controls expression of genes encoding key signaling, Janus kinase signal transducers and activators of transcription, mitogen-activated protein kinase, focal adhesion kinase, cell adhesion molecules, and cytokine-receptor interaction. It will be useful to determine whether these SRF targets are important for integrin trafficking. Equally important will be to examine in detail how SRF is regulated in response to chemokine and adhesion cues.
Overall, Taylor at al1 have uncovered a novel and important mechanism by which SRF controls neutrophil migration. SRF controls a program that leads to integrin clustering and trafficking, hence permitting efficient neutrophil adhesion and migration. The data reveal SRF’s specificity of function in neutrophils and add to the multifaceted roles of SRF function. It should be emphasized that integrin dysfunctions, either through their defective expression or activation, have been described in various forms of leukocyte adhesion deficiency in humans, leading to severe neutrophil-associated immunodeficiency.10 As noted, the SRF pathway is often deleted in del-5q MDS. Targeting the SRF pathway, either by enforcing its expression or target genes, could represent novel therapeutic opportunities for MDS or integrin-related immune disorders. Alternatively, because neutrophils are doubled-edge swords and contribute to tissue injury in pathological inflammation, inhibiting SRF pathways may provide novel options to limit neutrophil functions. Therefore, this study opens new research directions for identifying key mechanisms of neutrophil migration and for understanding the pathogenesis of human disease that may lead to novel therapeutic intervention in patients.
Conflict-of-interest disclosure: The author declares no competing financial interests.