Abstract
Recent studies of the anticoagulant activities of the tissue factor (TF) pathway inhibitor (TFPI) isoforms, TFPIα and TFPIβ, have provided new insight into the biochemical and physiological mechanisms that underlie bleeding and clotting disorders. TFPIα and TFPIβ have tissue-specific expression patterns and anticoagulant activities. An alternative splicing event in the 5′ untranslated region allows for translational regulation of TFPIβ expression. TFPIα has 3 Kunitz-type inhibitor domains (K1, K2, K3) and a basic C terminus, whereas TFPIβ has the K1 and K2 domains attached to a glycosylphosphatidyl inositol–anchored C terminus. TFPIα is the only isoform present in platelets, whereas endothelial cells produce both isoforms, secreting TFPIα and expressing TFPIβ on the cell surface. TFPIα and TFPIβ inhibit both TF–factor VIIa–dependent factor Xa (FXa) generation and free FXa. Protein S enhances FXa inhibition by TFPIα. TFPIα produces isoform-specific inhibition of prothrombinase during the initiation of coagulation, an anticoagulant activity that requires an exosite interaction between its basic C terminus and an acidic region in the factor Va B domain. Platelet TFPIα may be optimally localized to dampen initial thrombin generation. Similarly, endothelial TFPIβ may be optimally localized to inhibit processes that occur when endothelial TF is present, such as during the inflammatory response.
Tissue factor (TF) pathway inhibitor (TFPI) is the primary inhibitor of the initiation of blood coagulation and modulates the severity of a wide variety of bleeding and clotting disorders. TFPI impedes the early stages of the blood coagulation cascade through high-affinity inhibition of 2 coagulation proteases, TF–factor VIIa (TF-FVIIa)1-3 and factor Xa (FXa).2,3 This review is focused on the 2 major isoforms of TFPI: TFPIα and TFPIβ. These isoforms differ in their affinity for factor V/Va (FV/FVa)4,5 and protein S (PS),6-9 their tissue expression,10-13 their mechanism for association with cell surfaces,14-17 and their ability to impede early blood coagulation through inhibition of TF-FVIIa activity3 or inhibition of prothrombinase activity.5
Following vascular injury, TF is exposed to the blood and tightly binds the circulating serine protease FVIIa,18-20 increasing FVIIa catalytic activity by ∼30 000-fold.21,22 The TF-FVIIa complex initiates the blood coagulation cascade by activating factor X (FX) and factor IX (FIX).21,22 Additionally, factor IXa (FIXa) forms a complex with its cofactor protein, factor VIIIa (FVIIIa), which also activates FX.23 FXa binds its cofactor protein, FVa, to form prothrombinase, an enzymatic complex which rapidly converts prothrombin to thrombin.24,25 Thrombin then performs a number of procoagulant functions, including the activation of platelets,26 FV,27,28 factor VIII (FVIII),29,30 and factor XI,31,32 as well as cleavage of fibrinogen to fibrin.33
Deficiency of either FVIII or FIX manifests as the bleeding disorder hemophilia A or hemophilia B, respectively. Although the TF-FVIIa complex is known to activate FX directly, when small amounts of TF were added to FVIII- or FIX-deficient plasma, very little FXa was generated.34 This early experimental finding is consistent with the bleeding phenotypes observed in patients with hemophilia and suggested the presence of a plasma protein that inhibited TF-FVIIa from activating FX. Further studies showed that this inhibitory activity was reversible and required the presence of FVIIa, FXa, and calcium ions.35-41 These early studies led to the isolation and cloning of a coagulation inhibitor of TF-FVIIa–mediated activation of FX.42-46 This inhibitor was originally referred to as either the lipoprotein-associated coagulation inhibitor47 or the extrinsic pathway inhibitor,36 and eventually came to be known as TFPI. Two major isoforms of TFPI, resulting from an alternative splicing event, have now been described in both mice and humans: (1) TFPIα, which contains 3 Kunitz-type inhibitory domains (K1, K2, K3) and a positively charged C terminus46 and (2) TFPIβ, which contains the K1 and K2 domains of TFPIα, followed by a unique C terminus encoding for addition of a glycosylphosphatidylinositol (GPI) anchor.17,48 The K1 and K2 domains bind and inhibit FVIIa and FXa, respectively, whereas the K3 domain has no known inhibitory function.2
Clinical studies of TFPI
TFPI and thrombosis
Early studies using rabbit models demonstrated that immunodepletion of TFPI increases susceptibility to development of disseminated intravascular coagulation (DIC) following infusion of TF,49 evidence that TFPI is the primary regulator of TF-induced coagulation in vivo. Similarly, TFPI depletion increases susceptibility to endotoxin-induced DIC and the generalized Schwartzman reaction, characterized by fibrin deposition and hemorrhagic necrosis in the kidneys.50 Subsequently, genetically altered mice lacking the K1 domain of TFPI (TFPItm1Gjb; tfpi−/−) were produced and found to succumb to embryonic lethality from an apparent consumptive coagulopathy.51 Mice with heterozygous deficiency (tfpi+/−) demonstrate growth and development, reproductive capability, and survival comparable to tfpi+/+ mice.51 The tfpi+/− mice do not exhibit a generalized prothrombotic state under normal husbandry conditions, as evidenced by plasma thrombin-antithrombin complex levels comparable to tfpi+/+ mice.52 Furthermore, there is no difference between tfpi+/− and tfpi+/+ mice in time to occlusion following photochemical injury of the carotid artery,53 fibrin formation parameters measured by thromboelastography,54 or tail bleeding time.54 However, tfpi+/− mice have increased thrombus volume following femoral vein electrolytic injury, suggesting the presence of a mild procoagulant state.52 This mild procoagulant state is noticeably enhanced by breeding TFPI heterozygosity with other procoagulant mutations such as FV Leiden (F5tm2Dgi),55 apolipoprotein E deficiency (Apoetm1Unc/J),53 and partial thrombomodulin deficiency (Thbdtm1Wlr).52 Additionally, mice lacking only hematopoietic cell TFPI, which is primarily in platelets, also have increased clot volume following vascular injury,56 whereas mice lacking endothelial TFPI have decreased time to vascular occlusion following ferric chloride injury.57
Total TFPI deficiency has not been described clinically, suggesting that it also produces embryonic lethality in humans. Similar to what has been described in the murine system, partial TFPI deficiency also appears to be a rather weak prothrombotic risk factor in humans. Patients with plasma TFPIα (also called “full-length” or “free” TFPI) at or below the 10th percentile of the normal reference range were found to have slightly increased risk for venous thrombosis58 and an increased risk for coronary heart disease.59 Numerous other studies have correlated low plasma TFPI concentration with prothrombotic clinical disease, including atherosclerosis, coronary artery disease, ischemic stroke, and peripheral artery occlusive disease. These have been recently reviewed by Winckers and colleagues.60 Also of clinical interest is that oral estrogen therapy produces an ∼25% reduction in total plasma TFPI concentration and activity.61 It remains unclear how this may contribute to the procoagulant state associated with estrogen therapies. However, estrogen use appears to synergize with other risk factors. For example, women with FV Leiden who use oral contraceptives have greatly elevated thrombotic risk compared with women with only 1 of these risk factors.62 The severe perinatal thrombosis observed in mice with combined FV Leiden and heterozygous TFPI deficiency55 suggests a potential mechanism by which oral contraceptives decrease plasma TFPI concentration, reducing anticoagulant activity, and coupled with the procoagulant FV Leiden phenotype, this results in the elevated thrombotic risk.
The underlying reasons for the weak correlation between TFPI deficiency and thrombosis in humans are not entirely understood, but may lie in the assays available for clinical measurement of TFPI in plasma, which are complicated by several factors. Plasma TFPI can be measured either as TFPIα, which is not bound to lipoproteins, or as total TFPI, which is partially C-terminally truncated and associates tightly with lipoproteins. TFPIα, which contributes ∼10% to 30% of the total plasma TFPI pool, is thought to be the more active anticoagulant.63,64 The plasma concentration of TFPIα promptly increases twofold to fourfold following heparin infusion,15,16 suggesting that a large portion of TFPIα within the vasculature is associated with the endothelium and, therefore, is not measured in peripheral blood assays. This point is emphasized by patients with abetalipoproteinemia, who have very low amounts of plasma TFPI but normal amounts of heparin-releasable TFPI and do not have increased risk for thrombosis.65 In addition, as discussed in more detail in “Differential expression of TFPIα and TFPIβ in platelets and endothelial cells”, studies using cultured endothelial cells have identified an intracellular pool of TFPIα that is released following heparin treatment.66 Thus, measurement of plasma TFPI is likely not a reliable indicator of the intravascular TFPI anticoagulant activity present in an individual patient. TFPIα is also present within platelets.12,13 Development of assays that accurately measure platelet TFPI may provide a more reliable clinical indicator of intravascular TFPI activity and a patient’s risk for thrombotic disease.
TFPI and bleeding
The association of TFPI with bleeding disorders, such as hemophilia and a recently characterized mutation within the FV gene (east Texas bleeding disorder),67,68 has led to a greater understanding of the interdependent relationship between the procoagulant and anticoagulant mechanisms of hemostasis. The importance of TFPI as a modulator of bleeding in hemophilia could be hypothesized because, in its absence, the TF-FVIIa complex would generate increased FXa, even in the absence of FVIII or FIX, thus allowing for more significant thrombin production and clot formation. This hypothesis is supported by early studies, which showed that TFPI is an important regulator of coagulation in hemophilia A or B plasma69,70 and in a reconstituted system, containing physiologic concentrations of purified coagulation factors but lacking either FVIII or FIX.71 These in vitro studies suggest that the severe bleeding in patients with hemophilia requires not only the absence of FVIII or FIX, but also the presence of TFPI. This activity of TFPI is mediated by blocking direct activation of FX by TF-FVIIa, thereby requiring hemostatic thrombin generation to occur through activation of FIX and its cofactor, FVIII.34 Therefore, inhibition of TFPI activity has been investigated as a treatment strategy for hemophilia and has been demonstrated to improve hemostasis in several hemophilia animal models.54,72-76 One of these agents, BAX499,77,78 an aptamer which inhibits TFPIα72 by binding multiple sites including K1, K3, and the C terminus,79 initially showed promise as a novel therapeutic for treatment of hemophilia.80,81 However, in humans, it induced release of TFPIα from endothelial cells, stabilized the protein, and prevented its uptake and degradation, resulting in a substantial increase in TFPIα concentration (>25-fold under some conditions).77 The aptamer was not able to counteract the anticoagulant effect of this greatly elevated TFPI, bleeding resulted, and further development was stopped.78 Other pharmaceutical agents that block TFPI activity continue to be developed for treatment of hemophilia.76,82 Recent work from our laboratory demonstrated that deficiency of hematopoietic cell TFPI improved hemostasis in mice with FVIII deficiency.54 These data suggest that inhibition of FXa and prothrombinase on the platelet surface by TFPIα (discussed in “TFPIα and TFPIβ inhibit blood coagulation through distinct mechanisms”) may also contribute to bleeding severity in hemophilia and that pharmaceutical agents targeting only platelet TFPI released at a site of vascular injury may be sufficient to mitigate bleeding in patients with hemophilia.
TFPIα is also thought to modulate bleeding in FV-deficient patients.83,84 The C terminus of TFPIα interacts with FV.4 Patients with plasma FV deficiency are also deficient in plasma TFPIα, suggesting that these 2 proteins interact in plasma.84 This reduced TFPIα concentration allows for a small amount of thrombin generation in platelet-rich plasma from FV-deficient individuals, attributable to the low concentration of platelet FVa present in these patients.83 Therefore, it has been suggested that reduced TFPIα protects FV-deficient individuals from bleeding.83,84
The interaction between TFPIα and FV also contributes to the east Texas bleeding disorder, which is caused by an FV mutation (FVA2440G) that results in an alternative splicing event and removal of most of the FV B domain.67,68 This “FV-short” isoform retains the acidic region of its B domain,68 which tightly binds the TFPIα basic region.5 This interaction results in an ∼10-fold to 20-fold increase in circulating TFPIα (the reason for which is unknown but thought to reflect a stabilization of TFPIα by FV-short68 ) and produces a moderately severe bleeding phenotype characterized by bruising, epistaxis, and excessive blood loss following minor trauma, sometimes requiring blood transfusion for treatment.67,68 Very low concentrations of FV-short were also detected in the plasma of individuals without east Texas bleeding disorder, and TFPIα was shown to preferentially bind to FV-short compared with full-length FV.68 Therefore, FV-short may be the primary form of plasma FV that interacts with TFPIα under normal physiologic conditions, although this has not been directly demonstrated.
Alternative splicing produces TFPI isoforms with distinct structures
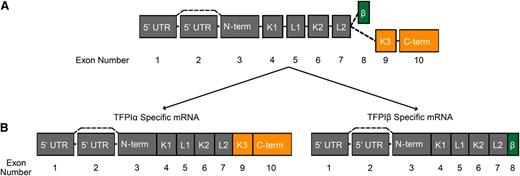
TFPI is an alternatively spliced protein with multiple, independent, splicing events occurring at both the 3′ and 5′ ends of the TFPI pre-messenger RNA (pre-mRNA) (Figure 1). Alternative splicing at the 3′ end produces several TFPI protein isoforms with distinct anticoagulant activities. The major isoforms, TFPIα and TFPIβ, are produced in all mammals. Other “minor” isoforms, identified only at the transcript level, include TFPIγ,85 produced only in mice, and TFPIδ,86,87 produced only in humans. The structure of these has been reviewed in detail elsewhere.86,87 The TFPIα and TFPIβ isoforms are differentially expressed in platelets and endothelial cells, respectively, and have different mechanisms for association with cell surfaces. TFPIα interacts with coagulation proteins FV/FVa and PS, ultimately producing unique mechanisms through which this isoform inhibits blood clotting. TFPIβ contains a GPI anchor attachment sequence and is directly bound to endothelium.17,48 The mRNA splicing pattern of TFPIα and TFPIβ is depicted in Figure 1. Exons 3 through 7 encode the N terminus, first and second Kunitz-type inhibitory domains (K1 and K2, respectively), and linker regions common to TFPIα and TFPIβ. Splicing to exons 9 and 10 produces TFPIα, which contains a third Kunitz domain (K3) that binds PS8 and a highly basic C terminus that binds FV/FVa.4,5 Splicing to exon 8 produces TFPIβ with its GPI anchor.17,48
Intron-exon organization of TFPI and the mRNA structure of TFPIα and TFPIβ. (A) Schematic diagram depicting the intron-exon organization of TFPI. (B) TFPIα and TFPIβ mRNA species. (A and B) Each box represents an exon; the joining lines represent introns. The exons are numbered below the diagrams. Dashed lines represent alternative splicing events. The region of TFPI encoded by each exon is indicated within the box. Exons encoding regions specific to TFPIα are colored orange; those specific to TFPIβ are colored green. β, region specific to TFPIβ; C-term, C terminus; K, Kunitz domain; L, linker region; N-term, N terminus.
Intron-exon organization of TFPI and the mRNA structure of TFPIα and TFPIβ. (A) Schematic diagram depicting the intron-exon organization of TFPI. (B) TFPIα and TFPIβ mRNA species. (A and B) Each box represents an exon; the joining lines represent introns. The exons are numbered below the diagrams. Dashed lines represent alternative splicing events. The region of TFPI encoded by each exon is indicated within the box. Exons encoding regions specific to TFPIα are colored orange; those specific to TFPIβ are colored green. β, region specific to TFPIβ; C-term, C terminus; K, Kunitz domain; L, linker region; N-term, N terminus.
Alternative splicing within the 5′ untranslated region (UTR) occurs in ∼12% of human mRNA species, including TFPI, and typically allows for inclusion or removal of translational regulatory elements.88 The 5′ UTR of TFPI is encoded by 2 exons (exons 1 and 2) that are present in TFPIα and TFPIβ message. Exon 2 is removed by alternative splicing in some transcripts.89,90 Recent data from our laboratory demonstrated that exon 2 acts as a translational repressor of TFPIβ, but not TFPIα, expression. Further experiments revealed that exon 2 is a general translational repressor whose repressive effects are overcome by elements within the TFPIα 3′ UTR. Thus, exon 2 splicing represents a “molecular switch” that regulates TFPIβ expression, providing a unique mechanism for regulation of TFPI isoform production, in which a 5′ alternative splicing event alters translation of a splice variant produced by a second splicing event at the 3′ end of the same pre-mRNA.90 The physiological importance of this 5′ splicing event remains to be determined but might include temporal and tissue-specific regulation of TFPIβ-mediated anticoagulant activity.
Differential expression of TFPIα and TFPIβ in platelets and endothelial cells
Endothelial cells and megakaryocytes are the primary cells producing TFPI.10-13 TFPI is also produced by monocytes and smooth muscle cells.91-96 Understanding the differential expression of TFPIα and TFPIβ within these different sites began by investigating their mRNA expression. In situ hybridization demonstrated that TFPIα and TFPIβ have the same cellular expression pattern, with the message for both primarily present in the vascular endothelium of tissues throughout the body.85 TFPIα mRNA is 5 to 10 times more abundant than TFPIβ mRNA in human and mouse tissues85 as well as cultured endothelial cells.97 Despite having less abundant mRNA, TFPIβ is the predominant TFPI isoform expressed on endothelium in murine tissues.11 TFPIβ is also the predominant isoform on cultured human endothelial cells and human placental microsomes, suggesting that TFPIβ is the predominant isoform on human endothelium, though cultured human endothelial cells also secrete TFPIα.98 It has been estimated that cultured human endothelial cells produce 10 to 50 times as much TFPIα as TFPIβ over a 24-hour period.98 Heparin infusion produces a prompt twofold to fourfold increase in human plasma TFPIα15,16,99,100 that is not observed in mice.11 This increase is observed within 5 to 10 minutes of heparin infusion, and is rapidly reversed following neutralization of heparin with protamine.99,100 C-terminally truncated forms of TFPIα produced in patients undergoing cardiac bypass surgery remain in the circulation after protamine infusion.99 Collectively, these data suggested that human endothelium has a heparin-releasable pool of TFPIα bound to cell-surface glycosaminoglycans through its basic C-terminal region. However, this in vivo pool of endothelial-associated TFPIα has been difficult to characterize using in vitro studies of cultured endothelial cells, which do not have a heparin-releasable form of TFPI on their surface.14,101,102 Instead, they have an intracellular store of TFPIα that is secreted as a soluble protein in a manner that can be enhanced by heparin, thrombin, or high shear force.66,103,104 Further investigation of human tissue vascular beds is needed to better define cell-surface TFPI isoform expression, as well as the presence and potential physiological function of the intracellular stores of TFPIα identified in cultured endothelial cells.
Human and mouse platelets make only TFPIα.12,56 TFPIα is stored within quiescent platelets and released following platelet activation.12,13 Interestingly, platelet TFPIα is secreted as a soluble protein following platelet activation with thrombin as a single agonist,13 with a portion becoming membrane-bound at the platelet surface following platelet activation with dual agonists, such as collagen plus thrombin.12 TFPIα does not colocalize with platelet α-granule or lysosomal proteins.12 Additional studies of its location within the platelet and the mechanism for its release are needed.
TFPIα and TFPIβ inhibit blood coagulation through distinct mechanisms
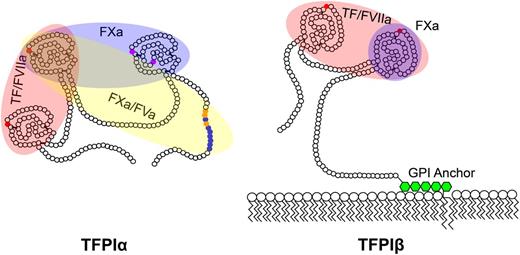
TFPIα and TFPIβ have distinct structural domains, mechanisms for association with cellular surfaces, and cellular expression patterns, suggesting they may yield anticoagulant activity through different biochemical mechanisms, at least some of which have been uncovered. TFPIα and TFPIβ contain the same K1 and K2 domains, which bind the active sites of FVIIa and FXa, respectively.2 Therefore, both isoforms are capable of directly inhibiting FXa2 and are FXa-dependent inhibitors of TF-FVIIa,1,2 as described when TFPI was initially identified and characterized2 (Figure 2). Very recently, our laboratory characterized a previously unrecognized TFPI anticoagulant activity, the inhibition of early forms of prothrombinase.5 This inhibitory activity is produced by TFPIα but not TFPIβ, and requires a high-affinity exosite interaction between the C-terminal basic region of TFPIα and an acidic region in the B domain of either FXa-activated FVa or forms of FVa released from platelet α-granules (Figures 2-3). Details of current knowledge of how TFPIα and TFPIβ produce anticoagulant activity, through TF-dependent and -independent mechanisms, are provided in the following paragraphs.
TFPI isoform structures. Shown are the amino acid structures of TFPIα and TFPIβ. Each small circle represents an individual amino acid. Small red circles indicate the residues in K1 and K2 which bind the active sites of FVIIa and FXa, respectively; small purple circles, residues in K3 which bind protein S; small blue and orange circles, the conserved basic and hydrophobic residues, respectively, which bind the acidic region of FVa. The domains required for inhibition of TF/FVIIa, FXa, and prothrombinase (FXa/FVa) are indicated by shaded red, blue, and yellow ovals, respectively. TFPIβ is anchored to the membrane through a GPI anchor (green hexagons).
TFPI isoform structures. Shown are the amino acid structures of TFPIα and TFPIβ. Each small circle represents an individual amino acid. Small red circles indicate the residues in K1 and K2 which bind the active sites of FVIIa and FXa, respectively; small purple circles, residues in K3 which bind protein S; small blue and orange circles, the conserved basic and hydrophobic residues, respectively, which bind the acidic region of FVa. The domains required for inhibition of TF/FVIIa, FXa, and prothrombinase (FXa/FVa) are indicated by shaded red, blue, and yellow ovals, respectively. TFPIβ is anchored to the membrane through a GPI anchor (green hexagons).
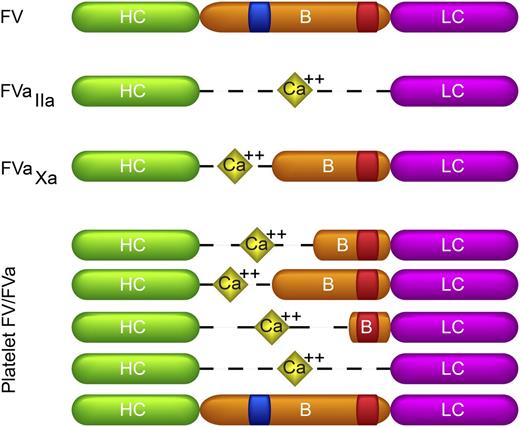
Biological forms of FV and FVa with different B-domain fragments. Shown are the domain structures of FV, thrombin-activated FVa (FVaIIa), FXa-activated FVa (FVaXa), and platelet FV/FVa, which is a mixture of multiple FVa species containing B domains of varying lengths, as well as full-length FV. The species shown are intended to indicate the heterogeneity and do not represent all of the platelet FVa species present or their relative abundance. The heavy chain (HC: green), light chain (LC: purple), and B domain (B: orange) are indicated. The heavy and light chains of FVa are linked through a calcium bridge (yellow diamonds). The basic and acidic regions of the B domain are indicated in blue and red, respectively. A form of FVa that contains the B-domain basic region but lacks the acidic region has not been identified.
Biological forms of FV and FVa with different B-domain fragments. Shown are the domain structures of FV, thrombin-activated FVa (FVaIIa), FXa-activated FVa (FVaXa), and platelet FV/FVa, which is a mixture of multiple FVa species containing B domains of varying lengths, as well as full-length FV. The species shown are intended to indicate the heterogeneity and do not represent all of the platelet FVa species present or their relative abundance. The heavy chain (HC: green), light chain (LC: purple), and B domain (B: orange) are indicated. The heavy and light chains of FVa are linked through a calcium bridge (yellow diamonds). The basic and acidic regions of the B domain are indicated in blue and red, respectively. A form of FVa that contains the B-domain basic region but lacks the acidic region has not been identified.
Inhibition of FXa by TFPIα and TFPIβ
TFPIα2 and TFPIβ3 directly inhibit FXa in assays which use small-molecule amidolytic substrates to measure FXa activity. The binding of PS to K3 in TFPIα and the GPI anchor that directly localizes TFPIβ to the cell surface are important differences between these 2 isoforms that alter their FXa inhibitory activity. TFPIα is a biphasic (slow, tight-binding) inhibitor of FXa and requires all Kunitz domains and the basic C terminus for optimal activity.2,8,64,105-110 PS enhances the inhibition of FXa approximately ninefold when studied using in vitro assays measuring FXa amidolytic activity.6,7 Evidence for the physiological importance of the PS-TFPIα anticoagulant system has accumulated through a series of clinical studies demonstrating that the plasma TFPIα concentration and anticoagulant activity are decreased in PS-deficient plasma, suggesting that PS and TFPIα circulate as a complex in plasma.111-113 Functional studies of the TFPIα-PS interaction have revealed that it requires K3 residues Arg1998 and Glu226114 and the presence of a membrane surface.6 Thus, it appears that PS enhances FXa inhibition primarily by localizing TFPIα to the membrane surface, a notion supported by experiments demonstrating that PS has cofactor activity toward the soluble TFPI variant protein K1K2K38,115 but has no cofactor activity toward the same variant protein when it is attached to the cell surface with a GPI anchor.115 In addition, PS does not enhance inhibition of FXa by cell surface–associated TFPI on primary endothelial cells or endothelial cell lines.115
Because optimal inhibition of FXa by TFPIα requires both the K3 domain and the basic C terminus8,64,107-110 (regions which are not present in TFPIβ), one might hypothesize that TFPIβ would be a relatively poor inhibitor of FXa. However, this is not the case. Membrane-associated TFPIβ is a slightly better inhibitor of FXa than is soluble TFPIα,3 suggesting that the GPI anchor circumvents the need for K3 and the C terminus in regards to inhibition of FXa by K2. As TFPIβ lacks K3, PS has no effect on its inhibitory activity.115 If the biological roles of K3, the C terminus, and PS during the inhibition of FXa are to bind TFPIα to the membrane surface, then it is logical that tethering TFPIβ to the surface through its GPI anchor would render K3, the C terminus, and PS unnecessary.
Inhibition of TF-FVIIa by TFPIα and TFPIβ
TFPIα1,2 and TFPIβ3,97,116,117 are FXa-dependent inhibitors of the TF-FVIIa complex, a process which is mediated by the interactions of K1 with the active site of FVIIa and K2 with the active site of FXa.2 The mechanism for inhibition of TF-FVIIa is often described as a 2-step process, with initial inhibition of FXa by K2 followed by inhibition of TF-FVIIa by K1118 ; however, TFPI most likely binds to the TF-FVIIa-FXa ternary complex, simultaneously inhibiting FXa and TF-FVIIa immediately after FX activation, with the binding of K2 to the FXa active site as the rate-limiting step.1 In contrast to its function as a cofactor for FXa inhibition (described in the previous section), the function of PS in the inhibition of TF-FVIIa by TFPIα has been less clear. PS was initially thought to promote the FXa-mediated inhibition of TF-FVIIa by TFPIα.6 However, further studies revealed that PS does not alter the rate of FXa or FIXa generation by TF-FVIIa.7 PS does promote the direct inhibition of FVIIa by K1, as measured using a small-molecule FVIIa substrate.9 This PS activity is dependent on TF, the TFPIα K3 domain, and phospholipid, suggesting that PS acts by localizing TFPIα to the membrane surface, in a manner similar to how it promotes FXa inhibition. Direct inhibition of TF-FVIIa by TFPIα may be physiologically relevant in contexts where TFPIα concentration is elevated, such as at the site of clot formation, where platelet TFPIα is released,12,13 or under pathologic conditions when plasma TFPIα is elevated.
TFPIβ also inhibits FXa generation by TF-FVIIa,3,97,116,117 as this activity only requires the K1 and K2 domains. In fact, TFPIβ inhibits the activity of TF-FVIIa as well as or better than TFPIα.3 TFPIβ is a more potent inhibitor of TF-FVIIa–mediated cellular migration in vitro and effectively dampens TF-mediated cellular infiltration into lung tissue in in vivo mouse model studies which used Chinese hamster ovary cells expressing either TF or both TF and TFPIβ.3 Similarly, suppression of TFPIβ expression in breast cancer cells results in enhanced cellular migration.116 One explanation for the enhanced inhibitory activity of TFPIβ compared with TFPIα would be that its expression on the same cell surface as TF resulted in a dramatically enhanced local concentration, which would suggest that TFPIβ is optimized to inhibit TF that is expressed on the same cell, as may occur under inflammatory conditions. As a GPI-anchored protein, TFPIβ might be expected to localize within cell-membrane microenvironments known as caveolae, as has been shown with other GPI-anchored proteins.119 Consistent with this, cell-surface TFPI on cultured cells localizes to caveolae, which enhance its anticoagulant activity.14,120-122 Studies of TFPIβ and altered membrane-bound forms of TFPI demonstrated that localization to caveolae does not alter direct inhibition of FXa by TFPIβ but does enhance its anti-TF activity.121 In addition, disruption of these membrane microdomains with methyl-β-cyclodextran results in decreased inhibitory activity.121
The TF-FVIIa-FXa ternary complex, apart from its role in coagulation, initiates signaling pathways through proteolytic activation of the protease activated receptors 1 and 2 (PAR1 and PAR2).123 Studies of the ability of TFPI to inhibit the signaling activity of the ternary complex have yielded mixed results.124 When TF activity is induced on human umbilical vein endothelial cells (HUVECs) by stimulation with tumor necrosis factor-α, the endogenously expressed HUVEC TFPI appears to be readily capable of inhibiting ternary complex-mediated signaling events, including PAR1-mediated induction of the orphan receptor gene TR3 and PAR2-mediated phosphorylation of extracellular signal-regulated kinase 1/2.124 As HUVECs express primarily TFPIβ on their surface,98 this inhibition was likely mediated by TFPIβ. In another set of experiments, HUVECs were transduced to overexpress TF and overwhelm the endogenous TFPI, and exogenous TFPIα was added.124 Under these conditions, minimal inhibition of PAR1-mediated signaling was observed, and inhibition of PAR2-mediated signaling also appeared to be weaker. These data suggest that TFPIβ is a more potent inhibitor of TF-FVIIa-FXa–mediated signaling than is TFPIα, similar to what has been reported for TF-FVIIa–mediated FX activation and cellular migration.3
Inhibition of prothrombinase by TFPIα
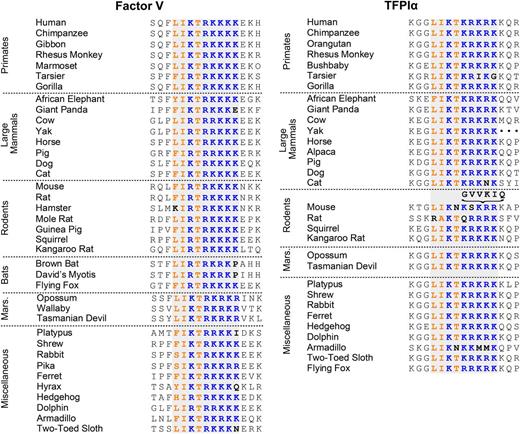
The inhibition of prothrombinase is a newly recognized, TF-independent, and isoform-specific anticoagulant activity of TFPIα.5 The ability of TFPIα to inhibit prothrombinase is dependent upon binding of its basic C-terminal region to an acidic region of the FV B domain that is retained in some forms of FVa that assemble into prothrombinase. Thus, TFPIα is a potent inhibitor of prothrombinase assembled with either FXa-activated FVa or platelet-released FVa, which retain the acidic region of the FV B domain,5 but not with thrombin-activated FVa, which has the entire B domain removed125,126 (Figure 3). This acidic region of the B domain interacts with a basic region, also within the B domain, to maintain FV in an inactive, procofactor conformation.127-129 These regions appear to function by preventing FXa from binding to the FV heavy chain, and removal of either the acidic or basic region is sufficient to relieve this inhibition and convert FV into FVa.127,128 Interestingly, the FV B-domain basic region contains an amino acid sequence almost identical to one found within the C terminus of TFPIα. This sequence is highly conserved in both proteins across mammalian species, suggesting that it performs an important physiological function (Figure 4). The TFPIα basic region binds to the acidic region within FXa-activated FVa and platelet-released FVa with high affinity (Kd ∼ 90pM) and, combined with the interaction between K2 and the FXa active site, allows TFPIα to inhibit prothrombinase containing either of these forms of FVa5 (Figure 5). This inhibitory activity is not shared by TFPIβ5 and is not enhanced by PS.115
Sequence alignment of the FV and TFPIα basic regions. Shown are residues 995 to 1010 of human FV and 249 to 264 of human TFPIα, along with the corresponding sequences from other mammalian species. The homologous basic region is shaded gray. The conserved basic residues are blue; the conserved hydrophobic residues are orange. Mouse TFPIα contains the indicated 6-residue insertion in the basic region. Sequences were obtained from the NCBI, UniProt, and OMA databases.
Sequence alignment of the FV and TFPIα basic regions. Shown are residues 995 to 1010 of human FV and 249 to 264 of human TFPIα, along with the corresponding sequences from other mammalian species. The homologous basic region is shaded gray. The conserved basic residues are blue; the conserved hydrophobic residues are orange. Mouse TFPIα contains the indicated 6-residue insertion in the basic region. Sequences were obtained from the NCBI, UniProt, and OMA databases.
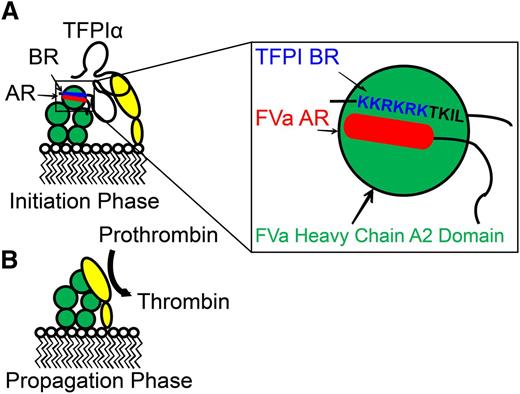
Mechanism of inhibition of prothrombinase by TFPIα. TFPIα inhibits thrombin generation by prothrombinase during the initiation phase of coagulation (A) but not during the propagation phase (B). This inhibition is mediated by the interaction of K2 with the active site of FXa (yellow), as well as the interaction of the TFPIα basic C terminus (blue) with the B-domain acidic region (red), present in FXa-activated FVa and some forms of platelet-released FVa (green). This region is absent in thrombin-activated FVa. Based on the model of Bos and Camire,127 it may be hypothesized that the TFPIα basic region and FVa B-domain acidic region bind to the FVa heavy chain A2 domain (expanded box). Modified from Wood et al5 with permission.
Mechanism of inhibition of prothrombinase by TFPIα. TFPIα inhibits thrombin generation by prothrombinase during the initiation phase of coagulation (A) but not during the propagation phase (B). This inhibition is mediated by the interaction of K2 with the active site of FXa (yellow), as well as the interaction of the TFPIα basic C terminus (blue) with the B-domain acidic region (red), present in FXa-activated FVa and some forms of platelet-released FVa (green). This region is absent in thrombin-activated FVa. Based on the model of Bos and Camire,127 it may be hypothesized that the TFPIα basic region and FVa B-domain acidic region bind to the FVa heavy chain A2 domain (expanded box). Modified from Wood et al5 with permission.
The TFPIα-mediated inhibition of prothrombinase is anticipated to be physiologically relevant and lead to a better understanding of and treatment of bleeding and clotting disorders. Forms of FVa that retain the acidic region of the B domain have been shown to have biological activity in 2 important recent studies. The first, performed by Schuijt and colleagues, used a tick anticoagulant protein (TIX-5) that specifically inhibits FXa from activating FV to demonstrate that FXa-catalyzed activation of FV is a critical event during the initiation of blood coagulation.130 The second, performed by Vincent and colleagues, characterized the east Texas bleeding disorder (mentioned in the “TFPI and bleeding” section), finding that the moderately severe bleeding in these patients is caused by tight binding of TFPIα to the acidic region of the B domain in the short form of FV produced in this disorder.68 Furthermore, TFPIα-mediated inhibition of prothrombinase is charge-dependent and thus is blocked by large, negatively charged molecules.5 This is consistent with previous studies which have shown that heparin, polyphosphate, and fucoidan have procoagulant properties in assays that rely on in situ generation of FVa (presumably through cleavage by FXa),74,125,131-133 and that heparin and polyphosphate lose these properties in the presence of thrombin-activated FVa.125,131,132 Thus, the inhibition of early forms of prothrombinase by TFPIα provides one explanation for the role of TFPIα in the procoagulant effects of heparin observed in the absence of antithrombin,125,131 the polyphosphate-mediated procoagulant activity of histone-stimulated platelet-rich plasma,134,135 and the efficacy of fucoidan administration in a dog model of hemophilia.75 It has also been proposed that the procoagulant effect of polyphosphate and heparin is due to acceleration of FV activation by either FXa or thrombin.131,132 This accelerated FVa generation allows for a more rapid assembly of prothrombinase, enabling FXa to escape inhibition by TFPI. It is likely that these compounds have multiple mechanisms of action.
Conclusions
TFPI was identified and cloned in the 1980s,42-46 with its mechanisms of FXa inhibition and FXa-dependent TF-FVIIa inhibition fully characterized through the 1990s.1,2,41,118 Subsequently, significant contributions have been made to the understanding of TFPI biology. We now know that there are 2 major TFPI isoforms produced in humans. TFPIα and TFPIβ arise from an alternative splicing event, are differentially regulated at the translational level, are expressed in different cell types, and are optimized for different anticoagulant functions. The GPI-anchoring of TFPIβ allows it to optimally inhibit TF-FVIIa and FXa on endothelium. TFPIα requires the aid of its cofactor PS to bind to cell surfaces and optimally inhibit FXa, though PS is not required for FXa-dependent TF-FVIIa inhibition. The C terminus of TFPIα allows it to inhibit early forms of prothrombinase, and its localization within platelets puts it in position to inhibit prothrombinase at the initial stages of clot formation. TFPI inhibitors are currently in development to treat bleeding disorders, including hemophilia, and our improved understanding of TFPI biology will enable us to better predict the physiologic effects of these inhibitors.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants HL068835 (A.E.M.), HL096419 and HL117702 (S.A.M.).
Authorship
Contribution: J.P.W., P.E.R.E., S.A.M., and A.E.M. wrote the manuscript.
Conflict-of-interest disclosure: A.E.M. receives grant support from Novo Nordisk. The remaining authors declare no competing financial interests.
Correspondence: Alan E. Mast, Blood Research Institute, Blood Center of Wisconsin, PO Box 2178, Milwaukee, WI 53201; e-mail: alan.mast@bcw.edu.