Key Points
Targeting the MUC1-C oncoprotein in MM cells potentiates BTZ-induced downregulation of TIGAR and thereby ROS-mediated death.
Targeting MUC1-C is effective in resensitizing BTZ-resistant MM cells to BTZ and thus represents a potential strategy for combination treatment.
Abstract
The proteosome inhibitor bortezomib (BTZ) induces endoplasmic reticulum and oxidative stress in multiple myeloma (MM) cells. The mucin 1 C-terminal subunit (MUC1-C) oncoprotein is aberrantly expressed in most MM cells, and targeting MUC1-C with GO-203, a cell-penetrating peptide inhibitor of MUC1-C homodimerization, is effective in inducing reactive oxygen species (ROS)-mediated MM cell death. The present results demonstrate that GO-203 and BTZ synergistically downregulate expression of the p53-inducible regulator of glycolysis and apoptosis (TIGAR), which promotes shunting of glucose-6-phosphate into the pentose phosphate pathway to generate reduced glutathione (GSH). In turn, GO-203 blocks BTZ-induced increases in GSH and results in synergistic increases in ROS and MM cell death. The results also demonstrate that GO-203 is effective against BTZ-resistant MM cells. We show that BTZ resistance is associated with BTZ-induced increases in TIGAR and GSH levels, and that GO-203 resensitizes BTZ-resistant cells to BTZ treatment by synergistically downregulating TIGAR and GSH. The GO-203/BTZ combination is thus highly effective in killing BTZ-resistant MM cells. These findings support a model in which targeting MUC1-C is synergistic with BTZ in suppressing TIGAR-mediated regulation of ROS levels and provide an experimental rationale for combining GO-203 with BTZ in certain settings of BTZ resistance.
Introduction
Multiple myeloma (MM) is a clonal malignancy of plasma cells that is characterized in part by the abnormal synthesis and secretion of monoclonal immunoglobulins or light chains.1 Cellular homeostasis is dependent on the balanced regulation of protein synthesis and degradation, the latter of which is predominantly mediated by the ubiquitin-proteosome pathway.2 Bortezomib (BTZ) is a reversible inhibitor of the proteosome that is effective in inducing apoptosis of MM cells and is active in the treatment of this disease.1 BTZ has improved response rates of MM patients to induction therapy and is being used as consolidation after frontline treatment or transplantation.1,3 However, intrinsic and acquired resistance to BTZ represent a challenge for the treatment of MM, which remains an incurable disease.1 BTZ has been shown to activate the unfolded protein response (UPR), a pathway induced by the accumulation of unfolded proteins in the endoplasmic reticulum (ER) and associated with increases in reactive oxygen species (ROS).4,5 In this way, BTZ treatment of MM cells induces expression of CCAAT/enhancer binding protein–homologous protein (CHOP; GADD153), a key transcription factor that participates in cellular responses to ER and oxidative stress.6-8 The mechanistic basis for BTZ activity has also been attributed to inhibition of inhibitory nuclear factor κB (NF-κB) degradation and thereby downregulation of the NF-κB pathway.9,10 In addition, mechanisms potentially unrelated to the UPR and NF-κB have been attributed to BTZ resistance. For example, mutations in the β5 proteosome subunit have been identified that decrease BTZ binding and sensitivity.11 Nonetheless, β5 subunit mutations have not been found in patients with BTZ resistance.12 Activation of phosphatidylinositol 3-kinase→protein kinase B signaling may also play a role in BTZ resistance in that inhibition of this pathway in MM cells contributes to BTZ sensitivity.13-15 Other studies of MM cells selected for BTZ resistance have demonstrated activation of the insulin-like growth factor-1 receptor (IGF-1R).16 In this regard, silencing IGF-1R or treatment with an IGF-1R inhibitor effectively resensitizes BTZ-resistant cell lines and patient samples to BTZ.16
Mucin 1 (MUC1) is a heterodimeric protein that is aberrantly expressed by most MM patient samples and cell lines.17-22 However, the functional significance of MUC1 expression in MM cells remains poorly understood. Certain insights into MUC1 function have evolved from the finding that MUC1 is translated as a single polypeptide which undergoes autocleavage into 2 subunits in the ER that, in turn, form a stable heterodimer at the cell surface.23 The MUC1 N-terminal subunit is positioned extracellularly in a complex with the transmembrane MUC1 C-terminal subunit (MUC1-C). The MUC1-C subunit includes a 72-amino-acid cytoplasmic tail that is phosphorylated by diverse kinases and thereby interacts with multiple effectors that have been linked to transformation.23,24 Moreover and in addition to its positioning at the cell membrane, MUC1-C is imported to the nucleus where it interacts with transcription factors that activate genes involved in growth and survival. MUC1-C also localizes to the mitochondrial outer membrane where it blocks the apoptotic response to stress. In concert with these functional roles, the MUC1-C subunit is sufficient for conferring anchorage-independent growth and tumorigenicity. In MM cells, silencing MUC1-C results in slowing of proliferation, enhanced sensitivity to apoptosis, and increased loss of self-renewal in the response to melphalan and dexamethasone, supporting involvement of MUC1-C in MM cell growth and survival.22 The MUC1-C subunit cytoplasmic domain includes a CQC motif that is necessary and sufficient for its homodimerization and oncogenic function.23,25 Accordingly, cell-penetrating peptides have been developed to target the CQC motif.26,27 The MUC1-C inhibitory peptides include the MUC1-C CQCRRKN sequence linked to 9 Arg residues for cell transduction, such that binding of the peptide to endogenous MUC1-C blocks its homodimerization.26-28 In particular, MUC1-C inhibitor treatment of MM cells growing in vitro and as tumor xenografts is associated with inhibition of growth and induction of late apoptosis/necrosis.29 Targeting MUC1-C in MM cells is also associated with (1) downregulation of the fructose-2-6-bisphosphatase p53-inducible regulator of glycolysis and apoptosis (TIGAR), (2) increases in ROS, and (3) decreases in reduced nicotinamide adenine dinucleotide phosphate (NADPH) and reduced glutathione (GSH) that contribute to cell death,30 indicating that MUC1-C functions in maintaining redox balance.
The present studies demonstrate that targeting MUC1-C with GO-203 in combination with BTZ promotes downregulation of TIGAR expression, decreases in GSH, and thereby cell death by an ROS-mediated mechanism. The results also show that (1) BTZ resistance is associated with increases in GSH levels, and (2) GO-203 reverses BTZ resistance by a TIGAR- and GSH-dependent mechanism. Targeting MUC1-C may therefore be effective alone and in combination with BTZ in certain forms of BTZ resistance.
Materials and methods
Cell culture
Human U266 and RPMI8226 MM cells were cultured in RPMI1640 medium (Cellgro) supplemented with 10% heat-inactivated fetal bovine serum (Cellgro), 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine. U266 and RPMI8226 cells were selected for BTZ resistance by exposure to increasing concentrations of this agent. BTZ-sensitive ANBL-6 and OPM-2, and their BTZ-resistant ANBL-6/BR and OPM-2/BR sublines, were cultured as described.16,31,32 Cells were treated with the MUC1-C inhibitor GO-203 ([R]9-CQCRRKN; dissolved at a stock concentration of 1 mg/mL in phosphate-buffered saline)29 or the inactive control peptide CP-2 ([R]9-AQARRKN) (AnaSpec), BTZ (LC Laboratories), and N-acetyl-cysteine (NAC; Calbiochem).
Immunoblot analysis
Cell lysates were prepared as described.29 Soluble proteins were analyzed by immunoblotting with anti-TIGAR (Abcam), anti-CHOP (Abcam), anti-activating factor 2 (ATF2) (Santa Cruz Biotechnology), anti-β-actin (Sigma-Aldrich), anti-caspase-8, anti-caspase-9, anti-poly ADP ribose polymerase (PARP) (Cell Signaling Technology), anti-protein kinase C δ (PKCδ) (Santa Cruz Biotechnology), and anti-β-catenin (BD Biosciences). Immune complexes were detected with horse-radish peroxidase–conjugated secondary antibodies and enhanced chemiluminescense (GE Healthcare).
Cell viability and apoptosis assays
Cell viability was assessed by staining with Alamar blue (Invitrogen). For assessment of cell death, cells were incubated with propidium iodide (PI)/annexin V–fluorescein isothiocyanate (BD Biosciences) for 15 minutes at room temperature and then analyzed by flow cytometry.
Measurement of ROS levels
For assessment of superoxide (O2−) levels, cells were incubated with 2 μM hydroethidine (Polyscience) for 30 minutes at 37°C. Superoxide-mediated conversion of hydroethidine to ethidium was measured in a flow cytometer at an excitation wavelength of 470 nm and an emission wavelength of 590 nm. Cells were incubated with 5 μM carboxy-2′7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (Molecular Probes) for 30 minutes at 37°C to assess hydrogen peroxide (H2O2)–mediated oxidation to 2′7′-dichlorodihydrofluorescein (DCF) as measured at an excitation wavelength of 480 nm and an emission wavelength of 590 nm as described.30
Measurement of GSH levels
Intracellular GSH concentrations were determined using the Bioxytech GSH-400 kit (OXIS International) as described.30
Determination of drug synergism
The combined effects of GO-203 and BTZ were determined by isobologram analysis using the CalcuSyn software program (Biosoft, Version 2.0). Using this approach, a combination index (CI) of <1.0 reflects synergism, and >1.0 indicates antagonism.
Statistical analysis
The Student t test was used to assess statistical significance.
Results
Targeting MUC1-C promotes BTZ-induced increases in ROS
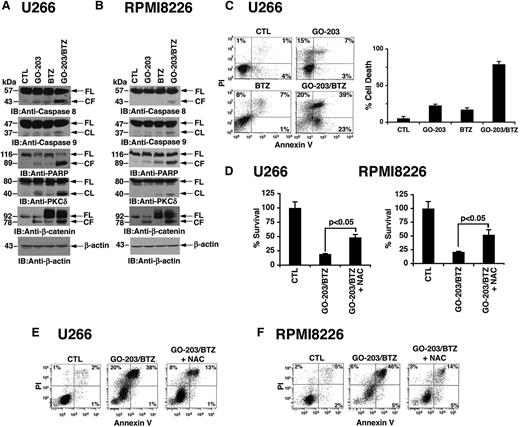
BTZ has direct inhibitory effects on the proteosome and, in turn, increases ROS levels that promote further decreases in proteosomal activity and thereby induce apoptosis.33,34 Blocking MUC1-C function in MM cells also induces ROS-mediated cell death.29,30 To determine whether targeting MUC1-C is effective in combination with BTZ, we treated cells with GO-203, a cell-penetrating peptide inhibitor of MUC1-C.30 GO-203 contains poly-Arg for membrane transduction linked to CQCRRKN that binds to the MUC1-C cytoplasmic domain CQC motif and blocks MUC1-C homodimerization (Figure 1A). Treatment of MM cells with GO-203 alone for 48 hours is associated with downregulation of TIGAR expression and thereby increases in ROS.30 Accordingly, U266 cells were treated with GO-203 for 48 hours, and then BTZ was added for an additional 24 hours (Figure 1B, left). Under these experimental conditions, BTZ alone had little if any effect on TIGAR levels (Figure 1B, left). However, the combination of GO-203 and BTZ was more effective in suppressing TIGAR expression than either agent alone. In addition, treatment with the antioxidant NAC blocked the decreases in TIGAR levels, indicating that this response is mediated by increases in ROS (Figure 1B, left). The demonstration that similar effects are observed in RPMI8226 cells treated with GO-203 and BTZ (Figure 1B, right) provided further support for an interaction between these agents in the regulation of TIGAR expression and induction of oxidative stress. TIGAR lowers cellular fructose-2,6-bisphosphate levels with inhibition of glycolysis and stimulation of the pentose phosphate pathway (PPP).35 These findings and the demonstration that exposure of MM cells to 5 μM GO-203 inhibits GSH production30 prompted studies to assess the effects of the GO-203/BTZ combination on GSH levels. Treatment with 2.5 μM GO-203 alone had little if any effect on GSH abundance in U266 (Figure 1C, left) and RPMI8226 (Figure 1C, right) cells. By contrast, BTZ treatment was associated with significant increases in GSH levels (Figure 1C, left and right). Moreover and significantly, the BTZ-induced increases in GSH were attenuated by GO-203 treatment (Figure 1C, left and right). In concert with these results, treatment of U266 cells with the GO-203/BTZ combination was more effective in increasing hydrogen peroxide (Figure 1D, left) and superoxide (Figure 1D, right) levels than that found with either agent alone. RPMI8226 cells also responded to the combination of GO-203 and BTZ with more pronounced increases in hydrogen peroxide (supplemental Figure 1A; see the Blood Web site) and superoxide (supplemental Figure 1B). BTZ treatment of MM cells is associated with increases in CHOP, a transcription factor that is induced in the response to ER and oxidative stress.6-8 Notably, treatment of U266 cells with GO-203, but not CP-2, was associated with the induction of CHOP expression (supplemental Figure 2A, left and right). CHOP levels were also increased in the response of RPMI8226 cells to GO-203 (supplemental Figure 2B, left and right). Moreover, the GO-203/BTZ combination was more active in inducing CHOP expression in both U266 (Figure 1E, left) and RPMI8226 (Figure 1E, right) cells as compared with GO-203 or BTZ alone. Treatment with GO-203, but not CP-2, was also associated with increased expression of ATF2 (supplemental Figure 2A-B), another transcription factor that is activated in the response to oxidative stress.36 In addition and as found for CHOP, the GO-203/BTZ combination was highly effective in inducing ATF2 expression (Figure 1E, left and right). These findings indicate that targeting MUC1-C promotes BTZ-mediated induction of oxidative stress.
Targeting MUC1-C in combination with BTZ downregulates TIGAR and induces oxidative stress. (A) Schema of the MUC1-C subunit with the amino acid sequence of the MUC1-C cytoplasmic domain. Highlighted is the CQC motif that is necessary and sufficient for MUC1-C homodimerization and is the target of GO-203. GO-203 consists of a cell-penetrating poly-Arg sequence ([R]9) upstream to CQCRRKN and binds to the MUC1-C cytoplasmic domain. CP-2 contains [R]9 upstream to AQARRKN and is inactive in MUC1-C binding. (B-E) The indicated cells were left untreated (control; CTL) and treated with (1) 2.5 μM GO-203 alone each day for 72 hours, (2) 9 nM BTZ alone for 24 hours, or (3) GO-203 for 48 hours combined with BTZ for an additional 24 hours. GO-203/BTZ-treated cells were also incubated in the presence of 5 mM NAC for 72 hours. Lysates were immunoblotted with the indicated antibodies (B,E). Cells were analyzed for relative GSH levels (mean ± standard deviation [SD] of 3 determinations) (C) and relative hydrogen peroxide or superoxide levels (mean ± SD of 3 determinations) as compared with that obtained with control cells (D).
Targeting MUC1-C in combination with BTZ downregulates TIGAR and induces oxidative stress. (A) Schema of the MUC1-C subunit with the amino acid sequence of the MUC1-C cytoplasmic domain. Highlighted is the CQC motif that is necessary and sufficient for MUC1-C homodimerization and is the target of GO-203. GO-203 consists of a cell-penetrating poly-Arg sequence ([R]9) upstream to CQCRRKN and binds to the MUC1-C cytoplasmic domain. CP-2 contains [R]9 upstream to AQARRKN and is inactive in MUC1-C binding. (B-E) The indicated cells were left untreated (control; CTL) and treated with (1) 2.5 μM GO-203 alone each day for 72 hours, (2) 9 nM BTZ alone for 24 hours, or (3) GO-203 for 48 hours combined with BTZ for an additional 24 hours. GO-203/BTZ-treated cells were also incubated in the presence of 5 mM NAC for 72 hours. Lysates were immunoblotted with the indicated antibodies (B,E). Cells were analyzed for relative GSH levels (mean ± standard deviation [SD] of 3 determinations) (C) and relative hydrogen peroxide or superoxide levels (mean ± SD of 3 determinations) as compared with that obtained with control cells (D).
GO-203 increases BTZ-induced cell death by an ROS-dependent mechanism
The induction of CHOP initially promotes recovery of ER function; however, failure to resolve ER stress, for example with further increases in ROS, results in the induction of apoptosis. Thus, to assess the effects of GO-203 and BTZ on cell death, we first studied activation of the extrinsic and intrinsic apoptotic pathways. Treatment of U266 cells with GO-203 alone resulted in a low level of caspase-8 cleavage (Figure 2A). A similar response was observed in the response to BTZ treatment (Figure 2A). Moreover, the GO-203/BTZ combination induced a marked increase in caspase-8 activation (Figure 2A), indicating that these agents induce the extrinsic apoptotic pathway. We also found that the GO-203/BTZ combination is effective in activating caspase-9 in the intrinsic apoptotic pathway (Figure 2A). Consistent with these results, the GO-203/BTZ combination was highly effective in inducing cleavage of the caspase-3 substrates, PARP, PKCδ, and β-catenin (Figure 2A). The demonstration that the GO-203/BTZ combination is also more effective in RPMI8226 cells than either agent alone in activating the extrinsic and intrinsic apoptotic pathways provided further support for their potential synergistic effects (Figure 2B). To extend this analysis, we assessed the effects of GO-203 and BTZ on the induction of cell death. As determined by PI/annexin V staining, the percentage of apoptotic/necrotic U266 cells was higher with the combination (82%) as compared with GO-203 (25%) or BTZ (16%) alone (Figure 2C, left). Analysis of repetitive experiments confirmed that the cell death response to the GO-203/BTZ combination is greater than that obtained for these agents alone (Figure 2C, right). Treatment of RPMI8226 cells with the GO-203/BTZ combination was also associated with a greater percentage of cell death than that observed with GO-203 or BTZ alone (supplemental Figure 3). Other studies have shown that death of MM cells in response to BTZ alone37,38 and GO-203 alone30 is reversed by NAC. In this context, we found that GO-203/BTZ induced decreases in survival of U266 (Figure 2D, left), and RPMI8226 (Figure 2D, right) cells are attenuated by NAC. As confirmation of these results, GO-203/BTZ-induced death of both U266 (Figure 2E) and RPMI8226 (Figure 2F) cells was attenuated by NAC treatment. These findings indicate that the GO-203/BTZ combination induces MM cell death, at least in large part, by an ROS-mediated mechanism.
GO-203 promotes BTZ-induced MM cell death. (A-E) U266 and RPMI8226 cells were left untreated (CTL) and treated with (1) 2.5 μM GO-203 alone each day for 72 hours, (2) 9 nM BTZ for 24 hours, or (3) GO-203 for 48 hours combined with BTZ during an additional 24 hours. Where indicated, GO-203/BTZ-treated cells were also incubated in the presence of 5 mM NAC for 72 hours. Lysates were immunoblotted with the indicated antibodies (A-B). U266 cells were incubated with PI and annexin V and analyzed by flow cytometry (C, left). The percentage of PI+ and/or annexin V+ cells is included in the panels (C, left). The results are expressed as the percentage (mean ± SD of 3 determinations) of dead cells (C, right). Percentage survival (mean ± SD of 3 determinations) was determined by Alamar blue staining (D). The indicated cells were incubated with PI and annexin V and analyzed by flow cytometry (E-F).
GO-203 promotes BTZ-induced MM cell death. (A-E) U266 and RPMI8226 cells were left untreated (CTL) and treated with (1) 2.5 μM GO-203 alone each day for 72 hours, (2) 9 nM BTZ for 24 hours, or (3) GO-203 for 48 hours combined with BTZ during an additional 24 hours. Where indicated, GO-203/BTZ-treated cells were also incubated in the presence of 5 mM NAC for 72 hours. Lysates were immunoblotted with the indicated antibodies (A-B). U266 cells were incubated with PI and annexin V and analyzed by flow cytometry (C, left). The percentage of PI+ and/or annexin V+ cells is included in the panels (C, left). The results are expressed as the percentage (mean ± SD of 3 determinations) of dead cells (C, right). Percentage survival (mean ± SD of 3 determinations) was determined by Alamar blue staining (D). The indicated cells were incubated with PI and annexin V and analyzed by flow cytometry (E-F).
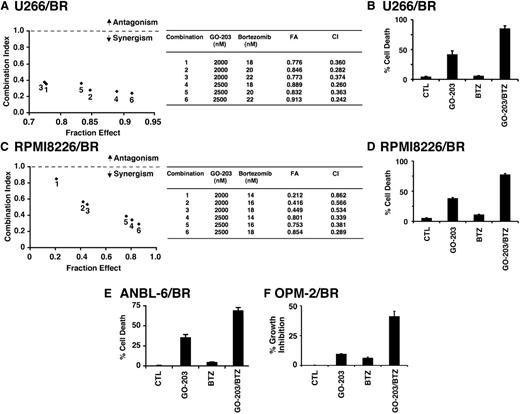
GO-203 is synergistic with BTZ and active in the setting of BTZ-resistant cells
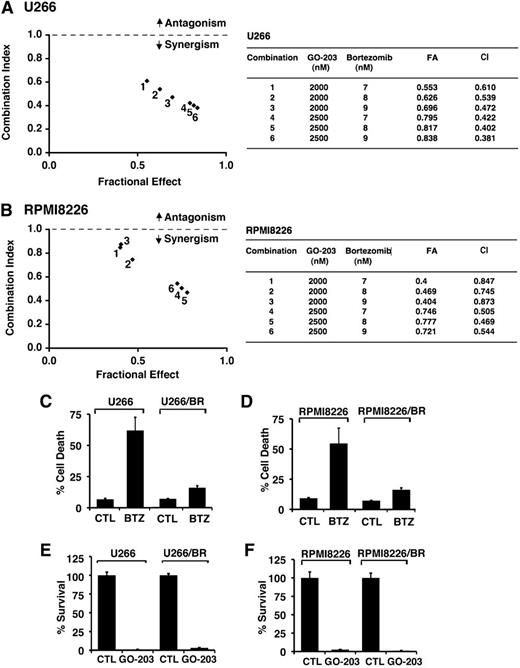
Given the previous findings, we examined whether the combination of GO-203 and BTZ at different doses of each agent induces synergistic anti-MM activity. U266 cells were thus treated with GO-203 at 2.0 and 2.5 μM and then with BTZ at 7, 8, or 9 nM (Figure 3A). Isobologram analysis of cell viability showed that these combinations are synergistic with CI values <1 (Figure 3A, left and right). A similar analysis of RPMI8226 cells treated with GO-203 and BTZ confirmed the synergistic interaction between these 2 agents (Figure 3B, left and right). The finding that GO-203 and BTZ exhibit synergistic activity prompted studies to determine whether targeting MUC1-C is affected in the setting of BTZ resistance. Accordingly, we grew cells in the presence of increasing BTZ concentrations to select for resistance. Using this approach, U266 cells resistant to BTZ (U266/BR) were selected for growth in the presence of 20 nM BTZ. Thus, treatment of drug-naïve U266 cells with 20 nM BTZ for 24 hours was associated with 61% cell death (Figure 3C). By contrast, similar treatment of U266/BR cells resulted in only 15% cell death (Figure 3C), supporting an approximately fourfold increase in resistance. In addition, RPMI8226 cells were selected for growth in the presence of 16 nM BTZ (Figure 3D). Treatment of drug-naïve RPMI8226 and RPMI8226/BR cells with 16 nM BTZ similarly resulted in 62% and 15% cell death, respectively (Figure 3D). Notably, however, the U266/BR (Figure 3E) and RPMI8226/BR (Figure 3F) cells remained sensitive to GO-203-induced inhibition of survival, providing support for the contention that targeting MUC1-C is synergistic in combination with BTZ and also active in the setting of BTZ resistance.
GO-203 is synergistic with BTZ and decreases survival of BTZ-resistant cells. U266 (A) and RPMI8226 (B) cells were treated with (1) the indicated concentrations of GO-203 alone each day for 72 hours, (2) the indicated concentrations of BTZ alone for 24 hours, and (3) GO-203 for 48 hours combined with BTZ for an additional 24 hours. Mean cell survival was assessed in triplicate by Alamar blue assays. Numbers 1 to 6 in the graphs (left) represent combinations listed in the tables (right). FA, fraction affected. (C-D) The indicated drug-naïve U266 and BTZ-resistant U266/BR (C) and drug-naïve RPMI8226 and BTZ-resistant RPMI8226/BR (D) cells were left untreated (CTL) and treated with 20 nM BTZ and 16 nM BTZ, respectively, for 24 hours. Cells were incubated with PI and annexin V and analyzed by flow cytometry. The results are expressed as the percentage (mean ± SD of 3 determinations) of dead cells. (E-F) The indicated cells were left untreated (CTL) and treated with 5 μM GO-203 each day for 72 hours. Percentage survival (mean ± SD of 3 determinations) was determined by Alamar blue staining.
GO-203 is synergistic with BTZ and decreases survival of BTZ-resistant cells. U266 (A) and RPMI8226 (B) cells were treated with (1) the indicated concentrations of GO-203 alone each day for 72 hours, (2) the indicated concentrations of BTZ alone for 24 hours, and (3) GO-203 for 48 hours combined with BTZ for an additional 24 hours. Mean cell survival was assessed in triplicate by Alamar blue assays. Numbers 1 to 6 in the graphs (left) represent combinations listed in the tables (right). FA, fraction affected. (C-D) The indicated drug-naïve U266 and BTZ-resistant U266/BR (C) and drug-naïve RPMI8226 and BTZ-resistant RPMI8226/BR (D) cells were left untreated (CTL) and treated with 20 nM BTZ and 16 nM BTZ, respectively, for 24 hours. Cells were incubated with PI and annexin V and analyzed by flow cytometry. The results are expressed as the percentage (mean ± SD of 3 determinations) of dead cells. (E-F) The indicated cells were left untreated (CTL) and treated with 5 μM GO-203 each day for 72 hours. Percentage survival (mean ± SD of 3 determinations) was determined by Alamar blue staining.
Targeting MUC1-C is effective against diverse BTZ-resistant cells
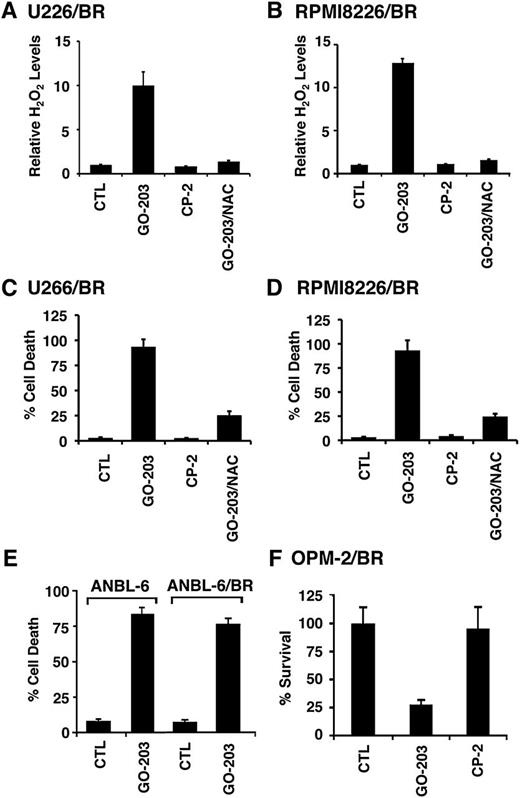
To further define the response of BTZ-resistant cells to MUC1-C targeting, we first compared ROS levels in drug-naïve U266 and U266/BR cells. The BTZ-resistant U266/BR cells exhibited a modest, but not significant, decrease in hydrogen peroxide (supplemental Figure 4A) and superoxide (supplemental Figure 4B) levels as compared with that in drug-naïve U266 cells. There were also no significant differences in hydrogen peroxide or superoxide levels when comparing RPMI8226 and RPMI8226/BR cells (supplemental Figure 4C-D), consistent with the tight control of redox balance by GSH and other antioxidants. Nonetheless, in further defining the response of BTZ-resistant cells to MUC1-C targeting, analysis of U266/BR cells treated with GO-203, but not CP-2, demonstrated a marked increase in hydrogen peroxide levels that was attenuated by NAC (Figure 4A). Similar results were obtained with GO-203-treated RPMI8226/BR cells (Figure 4B). The U266/BR and RPMI8226/BR cells also responded to GO-203, and not CP-2, with increases in superoxide levels (supplemental Figure 5A-B), indicating that targeting MUC1-C is effective in inducing ROS in both BTZ-sensitive and BTZ-resistant cells. In concert with the induction of ROS, GO-203 treatment of U266/BR (supplemental Figure 5C) and RPMI8226/BR (supplemental Figure 5D) cells was associated with caspase-8 activation and cleavage of PARP, PKCδ, and β-catenin. In addition and as found in drug-naïve cells, GO-203 treatment of U266/BR (Figure 4C) and RPMI8226/BR (Figure 4D) cells resulted in the induction of cell death. To confirm these results, additional BTZ-resistant MM cells were obtained from the Orlowski laboratory.16 GO-203 treatment of drug-naïve ANBL-6 and BTZ-resistant ANBL-6/BR cells16 was associated with the induction of similar increases in cell death (Figure 4E). BTZ-resistant OPM-2/BR cells16 also responded to GO-203, but not CP-2, with loss of survival (Figure 4F), indicating that targeting MUC1-C is effective against diverse types of BTZ-resistant MM cells.
BTZ-resistant MM cells respond to GO-203 with increases in ROS and cell death. (A-D) U266/BR (A) and RPMI8226/BR (B) cells were left untreated (CTL) and treated with 5 μM GO-203 or CP-2 each day for 72 hours. The GO-203-treated cells were also incubated in the presence of 5 mM NAC for 72 hours. Cells were analyzed for relative hydrogen peroxide levels (mean ± SD of 3 determinations) as compared with that obtained with control cells (A-B). Cells were incubated with PI and annexin V and analyzed by flow cytometry. The results are expressed as the percentage (mean ± SD of 3 determinations) of dead cells (C-D). (E) ANBL-6 and ANBL-6/BR cells were treated with 5 μM GO-203 each day for 72 hours, incubated with PI and annexin V, and analyzed by flow cytometry. The results are expressed as the percentage (mean ± SD of 3 determinations) of dead cells. (F) OMP-2/BR cells were left untreated (CTL) and treated with 5 μM GO-203 or CP-2 each day for 72 hours. Percentage survival (mean ± SD of 3 determinations) was determined by Alamar blue staining.
BTZ-resistant MM cells respond to GO-203 with increases in ROS and cell death. (A-D) U266/BR (A) and RPMI8226/BR (B) cells were left untreated (CTL) and treated with 5 μM GO-203 or CP-2 each day for 72 hours. The GO-203-treated cells were also incubated in the presence of 5 mM NAC for 72 hours. Cells were analyzed for relative hydrogen peroxide levels (mean ± SD of 3 determinations) as compared with that obtained with control cells (A-B). Cells were incubated with PI and annexin V and analyzed by flow cytometry. The results are expressed as the percentage (mean ± SD of 3 determinations) of dead cells (C-D). (E) ANBL-6 and ANBL-6/BR cells were treated with 5 μM GO-203 each day for 72 hours, incubated with PI and annexin V, and analyzed by flow cytometry. The results are expressed as the percentage (mean ± SD of 3 determinations) of dead cells. (F) OMP-2/BR cells were left untreated (CTL) and treated with 5 μM GO-203 or CP-2 each day for 72 hours. Percentage survival (mean ± SD of 3 determinations) was determined by Alamar blue staining.
Combining GO-203 and BTZ increases ROS in BTZ-resistant cells
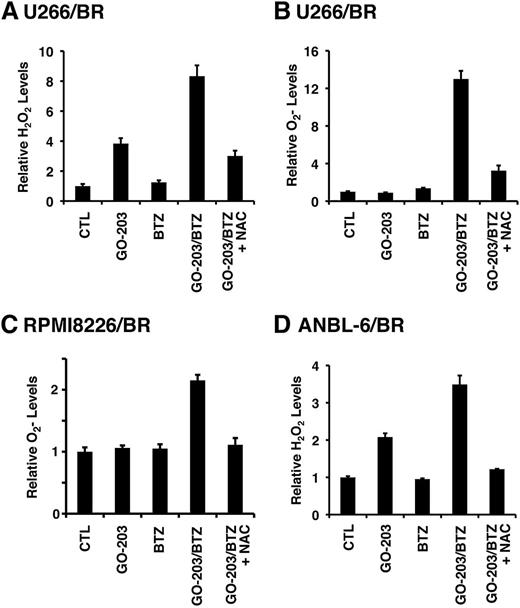
The demonstration that targeting MUC1-C increases ROS in BTZ-resistant cells prompted us to investigate the effects of combining GO-203 and BTZ. As expected, treatment of U266/BR cells with 20 nM BTZ had little if any effect on hydrogen peroxide levels (Figure 5A). However, the combination of GO-203 and BTZ was more effective in inducing hydrogen peroxide than either agent alone (Figure 5A). The GO-203/BTZ combination was also synergistic in increasing superoxide levels (Figure 5B). Similar responses were observed in RPMI8226/BR (Figure 5C) and MUC1-positive ANBL-6/BR (Figure 5D) cells treated with the combination of GO-203 and BTZ, indicating that these agents induce more than additive increases in ROS in these BTZ-resistant cells. Given the demonstration that drug-naïve MM cells respond to the GO-203/BTZ combination with induction of CHOP by an ROS-mediated mechanism (Figure 1E), we also analyzed CHOP levels in BTZ-resistant cells. Remarkably, CHOP expression was substantially downregulated in U266/BR, as compared with drug-naïve U266, cells (supplemental Figure 6A). CHOP expression was also decreased in RPMI8226/BR cells (supplemental Figure 6B), supporting a role for suppression of CHOP in association with BTZ resistance. Notably, however, and as found in drug-naïve cells, treatment of U266/BR (supplemental Figure 6C) and RPMI8226/BR (supplemental Figure 6D) cells with GO-203 and BTZ was associated with induction of CHOP expression that was suppressed by NAC, supporting an ROS-mediated mechanism. These findings indicate that the GO-203/BTZ combination is effective in inducing ROS and thereby CHOP in these BTZ-resistant cells.
GO-203 and BTZ synergistically induce ROS in BTZ-resistant cells. (A-D) The indicated cells were left untreated (CTL) and treated with (1) 2.5 μM GO-203 alone each day for 72 hours; (2) 20 (U266/BR), 16 (RPMI8226/BR) or 5 (ANBL-6/BR) nM BTZ for 24 hours; or (3) GO-203 for 48 hours combined with BTZ during an additional 24 hours. GO-203/BTZ-treated cells were also incubated in the presence of 5 mM NAC for 72 hours. Cells were analyzed for relative hydrogen peroxide (A,D) or superoxide levels (B-C) (mean ± SD of 3 determinations) as compared with that obtained with control cells.
GO-203 and BTZ synergistically induce ROS in BTZ-resistant cells. (A-D) The indicated cells were left untreated (CTL) and treated with (1) 2.5 μM GO-203 alone each day for 72 hours; (2) 20 (U266/BR), 16 (RPMI8226/BR) or 5 (ANBL-6/BR) nM BTZ for 24 hours; or (3) GO-203 for 48 hours combined with BTZ during an additional 24 hours. GO-203/BTZ-treated cells were also incubated in the presence of 5 mM NAC for 72 hours. Cells were analyzed for relative hydrogen peroxide (A,D) or superoxide levels (B-C) (mean ± SD of 3 determinations) as compared with that obtained with control cells.
Targeting MUC1-C sensitizes BTZ-resistant cells to BTZ treatment
The demonstration that GO-203 and BTZ are highly effective in increasing ROS in BTZ-resistant cells invoked the possibility that targeting MUC1-C might sensitize BTZ-resistant cells to BTZ killing. To address this line of reasoning, U266/BR cells were treated with GO-203 at 1.5, 2.0, and 2.5 μM each in combination with 18, 20, or 22 nM BTZ. Under these experimental conditions, the combinations were more effective in inhibiting growth than either agent alone (Figure 6A, left). Calculation of the CIs demonstrated a high degree of synergy with values of <1 (Figure 6A, right). U266/BR cells also responded to the GO-203/BTZ combination with a synergistic induction of cell death (Figure 6B). Similar findings were obtained from RPMI8226/BR cells treated with the GO-203/BTZ combination (Figure 6C-D). To extend this analysis, treatment of ANBL-6/BR cells with BTZ had little effect on cell viability (Figure 6E). However, the combination of GO-203 and BTZ was more effective in inducing cell death than that obtained with GO-203 alone, further indicating that GO-203 can reverse BTZ resistance (Figure 6E). The combination of GO-203 and BTZ was also synergistic in the induction of MUC1-positive OPM-2/BR cell death (Figure 6F). These findings collectively demonstrate that targeting MUC1-C sensitizes these BTZ-resistant cells to BTZ treatment.
GO-203 resensitizes BTZ-resistant cells to BTZ treatment. (A-D) U266/BR (A) and RPMI8226/BR (C) cells were treated with (1) the indicated concentrations of GO-203 alone each day for 72 hours, (2) the indicated concentrations of BTZ alone for 24 hours, and (3) GO-203 for 48 hours combined with BTZ for an additional 24 hours. Mean cell survival was assessed in triplicate by Alamar blue assays. Numbers in the graphs (left) represent combinations listed in the table (right). U266/BR (B) and RPMI8226/BR (D) cells were treated with 2.5 μM GO-203 alone, 20 (U266/BR) or 16 (RPMI8226/BR) nM BTZ alone, and the GO-203/BTZ combination. Cells were incubated with PI and annexin V and analyzed by flow cytometry. The results are expressed as the percentage (mean ± SD of 3 determinations) of dead cells. (E) ANBL-6/BR cells were left untreated (CTL) and treated with 2.5 μM GO-203 alone each day for 48 hours, 5 nM BTZ for 24 hours, or GO-203 for 24 hours combined with BTZ during an additional 24 hours. Cells were incubated with PI and annexin V and analyzed by flow cytometry. The results are expressed as the percentage (mean ± SD of 3 determinations) of dead cells. (F) OPM-2/BR cells were left untreated (CTL) and treated with 2.5 μM GO-203 alone each day for 72 hours, 7 nM BTZ for 24 hours, or GO-203 for 48 hours combined with BTZ during an additional 24 hours. Percentage growth inhibition (mean ± SD of 3 determinations) was determined by Alamar blue staining.
GO-203 resensitizes BTZ-resistant cells to BTZ treatment. (A-D) U266/BR (A) and RPMI8226/BR (C) cells were treated with (1) the indicated concentrations of GO-203 alone each day for 72 hours, (2) the indicated concentrations of BTZ alone for 24 hours, and (3) GO-203 for 48 hours combined with BTZ for an additional 24 hours. Mean cell survival was assessed in triplicate by Alamar blue assays. Numbers in the graphs (left) represent combinations listed in the table (right). U266/BR (B) and RPMI8226/BR (D) cells were treated with 2.5 μM GO-203 alone, 20 (U266/BR) or 16 (RPMI8226/BR) nM BTZ alone, and the GO-203/BTZ combination. Cells were incubated with PI and annexin V and analyzed by flow cytometry. The results are expressed as the percentage (mean ± SD of 3 determinations) of dead cells. (E) ANBL-6/BR cells were left untreated (CTL) and treated with 2.5 μM GO-203 alone each day for 48 hours, 5 nM BTZ for 24 hours, or GO-203 for 24 hours combined with BTZ during an additional 24 hours. Cells were incubated with PI and annexin V and analyzed by flow cytometry. The results are expressed as the percentage (mean ± SD of 3 determinations) of dead cells. (F) OPM-2/BR cells were left untreated (CTL) and treated with 2.5 μM GO-203 alone each day for 72 hours, 7 nM BTZ for 24 hours, or GO-203 for 48 hours combined with BTZ during an additional 24 hours. Percentage growth inhibition (mean ± SD of 3 determinations) was determined by Alamar blue staining.
BTZ resistance is associated with increases in GSH that are reversed by targeting MUC1-C
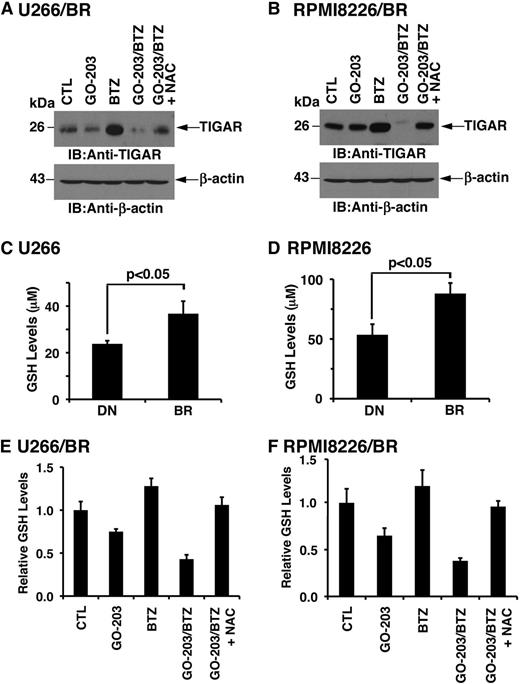
The finding that combining GO-203 and BTZ is synergistic against BTZ-resistant MM cells prompted an analysis of their effect on TIGAR expression. TIGAR levels were similar in the drug-naïve and BTZ-resistant MM cells (supplemental Figure 6A-B). In addition, using synergistic concentrations of these agents, treatment with GO-203 alone had little if any effect on TIGAR expression in U266/BR (Figure 7A) and RPMI8226/BR (Figure 7B) cells. By contrast, BTZ treatment was associated with increases in TIGAR levels (Figure 7A-B). Moreover and significantly, the BTZ-induced increases in TIGAR expression were substantially blocked by GO-203 treatment (Figure 7A-B). Subsequent experiments were therefore performed to assess effects on GSH levels in BTZ-resistant cells. Strikingly, a comparison of drug-naïve U266 and U266/BR cells demonstrated that BTZ resistance is associated with significant increases in GSH levels (Figure 7C). Analysis of RPMI8226/BR (Figure 7D), ANBL-6/BR (supplemental Figure 7A), and OPM-2/BR (supplemental Figure 7B) further demonstrated that selection for BTZ resistance results in significantly higher GSH levels. Treatment of U266/BR (supplemental Figure 8A) and RPMI8226/BR (supplemental Figure 8B) cells with GO-203 alone demonstrated suppression of GSH levels that are comparable to that observed in their drug-naïve counterparts. We therefore assessed the effects of combining GO-203 and BTZ on GSH levels in U266/BR cells. Here, GO-203 treatment resulted in GSH decreases that were more pronounced in combination with BTZ (Figure 7E). A similar effect of the GO-203/BTZ combination was observed in RPMI8226/BR cells (Figure 7F). These findings indicate that BTZ resistance is associated with increases in GSH and that GO-203 reverses the BTZ-resistant phenotype by downregulating TIGAR and decreasing GSH levels.
BTZ resistance is conferred by increases in GSH. (A-B) U266/BR (A) and RPMI8226/BR (B) cells were left untreated (CTL) and treated with (1) 2.5 μM GO-203 alone each day for 72 hours, (2) 20 (U266/BR) or 16 (RPMI8226/BR) nM BTZ for 24 hours, or (3) GO-203 for 48 hours combined with BTZ during an additional 24 hours. GO-203/BTZ-treated cells were also incubated in the presence of 5 mM NAC for 72 hours. Lysates were subjected to immunoblot analysis with the indicated antibodies. (C-D) The indicated drug-naïve and BTZ-resistant cells were analyzed for GSH levels. The results (mean ± SD of 3 determinations) are expressed as μM GSH per 106 cells. (E-F) U266/BR (E) and RPMI8226/BR (F) cells were treated as described in panels A and B and were analyzed for relative GSH levels (mean ± SD of 3 determinations) as compared with that obtained with control cells. BR, BTZ-resistant cells; DN, drug-naïve cells.
BTZ resistance is conferred by increases in GSH. (A-B) U266/BR (A) and RPMI8226/BR (B) cells were left untreated (CTL) and treated with (1) 2.5 μM GO-203 alone each day for 72 hours, (2) 20 (U266/BR) or 16 (RPMI8226/BR) nM BTZ for 24 hours, or (3) GO-203 for 48 hours combined with BTZ during an additional 24 hours. GO-203/BTZ-treated cells were also incubated in the presence of 5 mM NAC for 72 hours. Lysates were subjected to immunoblot analysis with the indicated antibodies. (C-D) The indicated drug-naïve and BTZ-resistant cells were analyzed for GSH levels. The results (mean ± SD of 3 determinations) are expressed as μM GSH per 106 cells. (E-F) U266/BR (E) and RPMI8226/BR (F) cells were treated as described in panels A and B and were analyzed for relative GSH levels (mean ± SD of 3 determinations) as compared with that obtained with control cells. BR, BTZ-resistant cells; DN, drug-naïve cells.
Discussion
Oxidation of cysteine residues as a result of disulfide bond formation in the ER contributes to oxidative stress and, in turn, activation of the UPR to attenuate oxidative protein folding.39 Treatment of MM cells with BTZ induces ER stress and thus UPR activation.4,5 The BTZ-induced UPR is associated with increases in ROS levels,7,37,38 which further promote ER stress and contribute to cell death.40,41 Targeting MUC1-C in MM cells is also associated with increases in ROS by a mechanism that involves downregulation of TIGAR,30 a fructose-2,6-bisphosphatase that lowers the activity of phosphofructokinase-1 and the glycolytic pathway.35,42 In this way, TIGAR increases NADPH and GSH levels and thereby decreases ROS.35 The present studies demonstrate that combining GO-203 with BTZ is highly effective in suppressing TIGAR expression, and, in turn, these agents synergistically increase both hydrogen peroxide and superoxide levels. Consistent with increases in ROS, the GO-203/BTZ combination was also effective in increasing expression of the ATF2 basic leucine zipper transcription factor, which is activated by oxidative stress43 and induces CHOP.6 CHOP contributes in part to cell death by inducing expression of the oxidase endoplasmic reticulum oxidoreductin-1α and thereby further disrupting redox balance in the ER.44,45 CHOP also triggers stress-induced apoptosis by activating expression of the Bcl-2 homology domain 3–only Bcl-2 interacting mediator of cell death protein.46 In concert with these findings, the GO-203/BTZ combination was more effective than either agent alone in inducing apoptotic cell death as evidenced by activation of the intrinsic and extrinsic pathways. In addition, PI/annexin V staining of GO-203/BTZ-treated cells demonstrated both apoptotic and necrotic cell death, which were ROS mediated as confirmed by their attenuation with NAC. These findings thus provide support for a model in which GO-203 and BTZ act in concert to suppress TIGAR, increase ROS, and induce cell death.
BTZ is an effective agent as a frontline treatment of patients with MM and in the relapsed/refractory disease settings.1 However, not all patients with MM respond to BTZ, and most of those who do respond ultimately relapse.1,47 These findings have stressed the importance of understanding the mechanisms underlying BTZ resistance and the identification of agents that are effective against relapsed/refractory disease. Studies of BTZ-resistant MM cell lines have demonstrated increased secretion of IGF-1 and activation of IGF-1R.16 In addition, treatment with OSI-906, an inhibitor of IGF-1R, is synergistic with BTZ and resensitizes BTZ-resistant cells to BTZ treatment.16 Of note, IGF-1R signaling has been linked to attenuation of oxidative stress in muscle cells48 ; however, subsequent studies will be needed to determine whether inhibiting IGF-1R in MM cells affects TIGAR and ROS. In the present work, BTZ-resistant MM cells were shown to be as sensitive to targeting MUC1-C as their drug-naïve counterparts. In addition and as found for drug-naïve MM cells, BTZ-resistant MM cells responded to GO-203 alone with increases in ROS and an induction of apoptotic/necrotic death that was attenuated by suppressing ROS with NAC. As expected, BTZ treatment of the BTZ-resistant MM cells had no effect on ROS levels. However, somewhat surprisingly, combining GO-203 with BTZ was associated with marked increases in ROS that were greater than that achieved with either agent alone. These results suggested that GO-203 resensitizes BTZ-resistant cells to BTZ-induced increases in hydrogen peroxide and superoxides. In this context, the GO-203/BTZ combination was synergistic in inhibiting growth and inducing apoptosis/necrosis of BTZ-resistant MM cells, confirming that GO-203 treatment reverses BTZ resistance. How targeting MUC1-C reverses BTZ resistance was not immediately apparent; however, the finding that NAC blocks this effect suggested that it is mediated by disrupting redox balance. Indeed, the available evidence indicates that ROS production and oxidative stress are integral UPR signals and that antioxidants can reduce ER stress.41 Accordingly, the GO-203-induced increases in ROS could resensitize BTZ-resistant cells to BTZ treatment by suppressing an antioxidant response that was acquired to reduce ER stress. Stated differently, BTZ resistance could be conferred at least in part by upregulation of antioxidant mechanisms, such as GSH, to attenuate the increases in ROS that contribute to BTZ-induced ER stress. In this way, attenuation of BTZ-induced upregulation of GSH promotes BTZ-induced cell death.49
Glutathione exists as reduced (GSH) and oxidized (GSSG) forms that are necessary for maintaining redox balance of the cell. Glutathione reductase reduces GSSG to GSH using NAPDH, which is generated by the PPP. Thus, TIGAR plays an important role in redox balance by increasing flux through the PPP and thereby generating NADPH for the reduction of GSSG.35 Previous work demonstrated that targeting MUC1-C in MM cells is associated with downregulation of TIGAR expression and marked decreases in NADPH and GSH.30 In addition, BTZ treatment has been associated with upregulation of GSH, and attenuation of this response promotes BTZ-induced cell death.49 The present studies demonstrate that, as found in drug-naïve MM cells, treatment of BTZ-resistant cells with GO-203 results in downregulation of GSH levels. Moreover and importantly, we found that BTZ-resistant MM cells have significant increases in GSH as compared with that in the drug-naïve setting, which based on the inhibitory effects of antioxidants would suppress BTZ-induced ER stress.41 In addition, we found that the BTZ-resistant phenotype is associated with downregulation of CHOP by a redox-dependent mechanism. These and our other findings therefore support a model in which GO-203 treatment blocks the increases in GSH that are associated with BTZ resistance. The present results also demonstrate that GO-203 and BTZ are synergistic in decreasing TIGAR expression and GSH levels, lending further support for this response in contributing to their synergy in killing BTZ-resistant cells. How GO-203 and BTZ suppress TIGAR levels will require additional study. TIGAR expression is downregulated by a posttranscriptional ROS-mediated mechanism.30 Thus, TIGAR protein, but not messenger RNA, is decreased by oxidative stress, and this response is abrogated by antioxidants, such as NAC.30 Other studies have shown that TIGAR messenger RNA levels are downregulated by oxidative stress.50 TIGAR also localizes to mitochondria, forms a complex with hexokinase 2, and increases hexokinase 2 activity.51 Involvement of TIGAR in promoting the PPP is independent of its mitochondrial localization; nonetheless, both functions contribute to limiting ROS levels and protecting against cell death.51 Therefore, the effects of the GO-203/BTZ combination on suppression of TIGAR expression could disrupt redox balance by both of these TIGAR-mediated pathways.
Finally, a phase 1 trial of GO-203 for patients with refractory solid tumors has been completed with the identification of a maximum tolerated dose for phase 2 studies. Pharmacokinetic studies have shown that plasma GO-203 levels are achieved at the low micromolar concentrations used in the present in vitro experiments. The demonstration that GO-203 is effective in inducing death of MM cells growing in vitro and as tumor xenografts29,30 has supported the evaluation of this agent in patients with MM. The present findings that targeting MUC1-C promotes BTZ-induced death and reverses certain forms of BTZ resistance provide further support for combining GO-203 with BTZ in the treatment of MM. In this context, a phase 1b/2a trial is planned in which patients with relapsed/refractory MM will be treated with GO-203 alone and in combination with BTZ.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Robert Orlowski, MD Anderson Cancer Center, for providing the BTZ-resistant MM cells.
This work was supported by the National Cancer Institute of the National Institutes of Health (CA100707 and CA42802) and by the Leukemia Lymphoma Society (6226-12).
Authorship
Contribution: L.Y. and T.K. performed research and analyzed data; D.A. and D.K. designed research and analyzed data; and D.K. wrote the manuscript.
Conflict-of-interest disclosure: D.K. holds equity in Genus Oncology and is a consultant to the company. The remaining authors declare no competing financial interests.
Correspondence: Donald Kufe, 450 Brookline Ave, Dana 830, Boston, MA 02215; e-mail: donald_kufe@dfci.harvard.edu.

![Figure 1. Targeting MUC1-C in combination with BTZ downregulates TIGAR and induces oxidative stress. (A) Schema of the MUC1-C subunit with the amino acid sequence of the MUC1-C cytoplasmic domain. Highlighted is the CQC motif that is necessary and sufficient for MUC1-C homodimerization and is the target of GO-203. GO-203 consists of a cell-penetrating poly-Arg sequence ([R]9) upstream to CQCRRKN and binds to the MUC1-C cytoplasmic domain. CP-2 contains [R]9 upstream to AQARRKN and is inactive in MUC1-C binding. (B-E) The indicated cells were left untreated (control; CTL) and treated with (1) 2.5 μM GO-203 alone each day for 72 hours, (2) 9 nM BTZ alone for 24 hours, or (3) GO-203 for 48 hours combined with BTZ for an additional 24 hours. GO-203/BTZ-treated cells were also incubated in the presence of 5 mM NAC for 72 hours. Lysates were immunoblotted with the indicated antibodies (B,E). Cells were analyzed for relative GSH levels (mean ± standard deviation [SD] of 3 determinations) (C) and relative hydrogen peroxide or superoxide levels (mean ± SD of 3 determinations) as compared with that obtained with control cells (D).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/19/10.1182_blood-2013-11-539395/4/m_2997f1.jpeg?Expires=1767842181&Signature=SQhzWgTd16deq8Ks7Ig4cy0TiuwS6sX5huMCu0QwcAc13jA1-DaQrR7LiDeIN94YPDx5vZzXTiGx5sndzMhcnkGN8V9fi-pIxuILFnnZcn1UQiLcsHa2y5UhJNhgH91s-lHzJeNQyQSp3Hf2~wgzBPZPKw9U~PINlVbmjR4OzkH1DGod0Tfq1zXW1T7XvCzA5hRLMXazy~svOJFSPLTzVMsTZcx7BEN3sT3w04bQDG8WCZbvrjW8M-4zUz1r554PUkCnrk0IlKUhbyWD-r-YRcDSKfiSFJAWm-TAI0M~YQqxHGemKyV1ulEh6BNoU8N2MRExYHwoGm2KK1Ifkrhh0w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal