Key Points
PML4 promotes erythroid differentiation in K562, primary erythroid cells, and GATA-1–rescued G1E-ER4 cells, but not in GATA-1–deficient G1E cells.
PML4 interacts with GATA-1 and enhances its transcriptional activity by stimulating GATA-1/P300 cooperation and GATA-1 acetylation.
Abstract
Promyelocytic leukemia protein (PML) has been implicated as a participant in multiple cellular processes including senescence, apoptosis, proliferation, and differentiation. Studies of PML function in hematopoietic differentiation previously focused principally on its myeloid activities and also indicated that PML is involved in erythroid colony formation. However, the exact role that PML plays in erythropoiesis is essentially unknown. In this report, we found that PML4, a specific PML isoform expressed in erythroid cells, promotes endogenous erythroid genes expression in K562 and primary human erythroid cells. We show that the PML4 effect is GATA binding protein 1 (GATA-1) dependent using GATA-1 knockout/rescued G1E/G1E-ER4 cells. PML4, but not other detected PML isoforms, directly interacts with GATA-1 and can recruit it into PML nuclear bodies. Furthermore, PML4 facilitates GATA-1 trans-activation activity in an interaction-dependent manner. Finally, we present evidence that PML4 enhances GATA-1 occupancy within the globin gene cluster and stimulates cooperation between GATA-1 and its coactivator p300. These results demonstrate that PML4 is an important regulator of GATA-1 and participates in erythroid differention by enhancing GATA-1 trans-activation activity.
Introduction
Erythropoiesis is a multistep process that includes erythroid commitment, proliferation, and erythrocyte maturation. GATA binding protein 1 (GATA-1) is a master erythroid transcription factor, as its function appears to be essential for almost all aspects of erythropoiesis. GATA-1 knockout mice suffer from fatal anemia at day 10.5 of gestation as a consequence of the failure of erythroid cells to survive and mature.1 GATA-1 engages DNA and coregulatory proteins through a dual-zinc finger domain and regulates almost all erythroid-specific genes.2 The trans-activation activity of GATA-1 is modulated by multiple, well-characterized cofactors (such as friend of GATA [FOG] 1, mediator of RNA polymerase II transcription subunit 1 [TRAP] 220, CREB-binding protien [CBP/p300], and HDAC5) during erythropoiesis.3 In addition, GATA-1 undergoes multiple posttranslational modifications, including phosphorylation, sumoylation, and acetylation, which also regulate GATA-1 activity. Acetylation of GATA-1 by acetyltransferases CBP/p300 significantly stimulates its transcriptional activity and regulates its chromatin occupancy in vivo.4,5
Promyelocytic leukemia protein (PML), initially identified in the leukemogenic PML/retinoic acid receptor translocation, is an essential component of the nuclear PML body in which many transcriptional factors and post-translational enzymes colocalize.6,7 PML participates in diverse cellular processes, including apoptosis, the DNA damage response, senescence, and differentiation, in part by providing colocalized transcriptional factors a platform for efficient post-translational modification. PML acts as a transcriptional cofactor of p53 and enhances its activity in promoting cell apoptosis and senescense, which is in part dependent on its ability to bind and recruit CBP/p300 or histone deacetylase into PML bodies.8-10 Seven alternatively spliced PML isoforms have been identified, each of which shares a common N-terminal RING finger, B-box, and Coiled-Coil domain (RBCC) motif, but differ in their C-terminal isoforms.11,12 Specifically, PML4 has been reported to promote the association of transcription factor PU.1 and p300, thereby enhancing PU.1-induced transcription and granulocytic differentiation.13 Moreover, PML4 increases granulocytic differentiation of U937 cells by interacting with and destabilizing c-Myc.14 One report also connected PML to erythroid differentiation by showing that PML expression increased and was sustained during erythroid differentiation of human hematopoietic progenitor cells (HPCs) and that inhibition of PML caused diminished HPC-derived erythroid colony formation.15 The process was shown to involve pRb, a general transcriptional factor essential for terminal differentiation of multiple cell types. However, the precise role played by PML in erythroid differentiation and the underlying molecular mechanisms for which it is responsible remain largely unknown.
In this study, we report that PML promotes erythroid gene expression and erythroid differentiation in an isoform-specific and GATA-1–dependent manner. PML4, but not other detected PML isoforms, interact directly with GATA-1 and enhance its transcriptional activity. Furthermore, we found that PML4 enhances the chromatin occupancy of GATA-1 at regulatory binding sites and stimulates GATA-1/p300 cooperation and GATA-1 acetylation. Our experiments implicate an important role for PML in GATA-1–mediated transcription and erythroid differentiation.
Methods
Plasmid constructs and antibodies
Full-length and deletion-mutant PML4 and GATA-1, as well as the PML1+4L and PML1+4S chimeric molecules, were obtained by reverse transcriptase-polymerase chain reaction (RT-PCR) and cloned into pCMV-hemagglutinin (HA) (Clontech) or pcDNA4/myc-His (Invitrogen), respectively. The expressing plasmids for human PML isoforms (PML1-3) were kindly provided by Dr Kun-Sang Chang (University of Texas, Houston, TX). The full-length PML1, PML4, and GATA-1 were then subcloned into retrovirus vector pMSCV-neo (Clontech) for gene transfer of erythroid cell lines and primary erythroid cells. For in vitro protein expression, cDNAs of PML4 and GATA-1 were subcloned into pMAL-4X (New England Biolab) and pET-42a (Novagen), respectively.
Antibodies against PML (PG-M3, sc-966, H-238, and sc-5621), GATA-1 (N6 and sc-265), c-Myc (9E10 and sc-40), and p300 (N-15 and sc-584) were obtained from Santa Cruz Biotechnology. Anti-CD235 (glycophorin A [GPA])-PE (No.12-9987-80) and control immunoglobulin (Ig)G2b-PE (No.12-4732-41) were obtained from eBioscience. Anti–GATA-1 antibody for co-immunoprecipitation (Co-IP) assays in primary erythroid cells were from Proteintech (No. 60011-1). HA and β-actin monoclonal antibodies were from Sigma-Aldrich.
Cell culture
HeLa, 293T, and MEL cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen). HEK-293A cells were grown in α-minimum essential medium (Invitrogen). K562 cells were cultured in RPMI 1640 medium (Invitrogen). All media were supplemented with 10% fetal bovine serum (Invitrogen). For K562 and MEL cells, erythroid differentiation was induced by addition of 50 μM hemin and 2% dimethylsulfoxide, respectively. G1E and G1E-ER4 cells were a generous gift from M. J. Weiss (Children’s Hospital, Philadelphia, PA) and were induced to differentiate with 10−7 M β-estradiol (Sigma-Aldrich) as described.16,17 Primary human CD34+ cells were obtained from magnetically sorted mononuclear of umbilical cord blood from donors with acquired consent in accordance with the Declaration of Helsinki (institutional review board approval number 021-2011; Institute of Basic Medical Sciences, Peking Union Medical College) and differentiated into an erythroid lineage as described with small modifications.18
RNA interference and retrovirus package
Cell lines stably expressing short hairpin RNAs (shRNAs) of PML and green fluorescent protein (GFP; control) were established using vector-based shRNA expression system. The shRNA targeting PML or GFP was inserted into pSIREN-retroQ (Clontech) with the following sequences: shPML, 5′-GAGUCGGCCGACUUCUGGU-3′,19 and shGFP, 5′-GCAAGCTGACCCTGAAGTT-3′. The plasmids were transfected into 293T cells along with packaging plasmids (pMD and pVSV-G), and viral supernatant was collected to infect target cells as described in the supplier’s protocol. Forty-eight hours after infection, 1 μg/mL puromycin was added for ∼7 to 10 days to generate stable knockdown cell lines. PML knockdown efficiency was determined by RT-PCR and immunoblotting.
IP and glutathione S-transferase pull-down
Cells were collected and solubilized in IP buffer (1% NP-40, 150 mM NaCl, 50 mM Tris-HCl [pH 7.4], 0.25% sodium deoxycholate, 1 mM EDTA, and protease inhibitors) at 4°C. Lysed protein mixtures were incubated with specific antibodies and precipitated with protein A or G agarose (Upstate Biotechnology) at 4°C overnight. The immunocomplex was collected, washed, and probed with designated antibodies. For glutathione S-transferase pull-down, GST fusions (GST-GATA-1 and control GST) and maltose binding protein (MBP)-tagged PML4 from bacterial lysates were purified, and binding assays in vitro were carried out using glutathione sepharose (GE Healthcare) according to the manufacturer’s instructions. Precipitated proteins or total lysates were separated by 12% polyacrylamide gel electrophoresis followed by immunoblotting.
Chromatin IP
The chromatin IP (ChIP) assay was performed essentially as previously described.20 For quantification of the ChIP assay, real-time PCR was performed with SYBR Green dye on an iCycler iQ (Bio-Rad) system. All quantitative PCR signals from IP samples were normalized to that of respective input samples. Values shown are the means and standard deviations of the results from ≥3 independent experiments. All primer sets are available on request.
See supplemental Data on the Blood Web site for the methodology of real-time PCR, reporter assays, electrophoretic mobility shift assay, and cell cycle assays.
Results
PML4 promotes terminal erythroid differentiation in a GATA-1–dependent manner
To understand the role of PML in erythroid differentiation, we first examined the effect of PML on endogenous erythroid gene expression. In K562 cells, stimulating PML expression by interferon (IFN) activates the expression of most globin genes (supplemental Figure 1). Forcible overexpression of PML4, the most studied PML isoform, in K562 cells leads to increased expression of the globin genes, as well as α-hemoglobin stabilizing protein (AHSP) (Figure 1A-B), accompanied by a significantly elevated ratio of GPA-positive to -negative (immature) cells (Figure 1C). Transcriptional profiling of the cells shows changes in GATA-1 and erythropoietin (EPO) signaling pathways and in other nonerythroid regulators (supplemental Figure 2; supplemental Table 1). We also detected G0/G1 cell cycle arrest and expression changes of c-myc and p27 in K562 cells that ectopically expressed PML4 (supplemental Figure 3). Consistently, knockdown of PML expression in K562 cells with retroviral siRNA19 resulted in reduced globin and AHSP transcription (Figure 1D-E; supplemental Figure 4A) and an obvious drop in GPA-positive cells (Figure 1F). Interestingly, overexpression of PML1, the longest isoform of PML, did not promote expression of erythroid genes (supplemental Figure 5).
PML4 promotes terminal erythroid differentiation through GATA-1. (A-C) Forced expression of PML4 promotes K562 erythroid differentiation. K562 cells were infected with a retrovirus that expressed PML4 cDNA. (A) Western blot was performed to detect overexpression of PML. (B) Real-time RT-PCR was performed for AHSP and globin gene expression. (C) Flow cytometry was used to detect expression of GPA. (D-F) PML knockdown in K562 cells leads to reduced expression of erythroid genes. K562 cells were infected with a retrovirus harboring an shRNA to knock down PML (or GFP as a control). (D) Western blot assay, (E) real-time RT-PCR, and (F) flow cytometry were performed as in A-C. (G) Human primary erythroid progenitors were expanded and induced to erythroid differentiation by EPO. At day 1 of differentiation, retroviral vectors for PML4 overexpression or PML knockdown were transfected. PML and β-like globin genes expression were determined on day 4 of differentiation. (H-J) PML4 promoted erythroid maturation of G1E-ER4 but not G1E. PML4 and control plasmids were stably overexpressed in G1E and G1E-ER4 cells by retrovirus infection and induced to erythroid differentiation by adding β-estradiol for 18 hours of treatment. (H) Western blotting analysis of GATA-1 and PML4 expression in PML4 overexpressing and control G1E, G1E-ER4 cells before and after β-estradiol induction. (I) Real-time RT-PCR analysis of AHSP and α- and β-globin genes expression in PML4 overexpressing and control G1E and G1E-ER4 cells before and after β-estradiol induction. Right panel shows the enlarged bars from G1E cells. (J) Benzidine staining of PML4 overexpressing and control G1E-ER4 cells before and after 18 hours of β-estradiol induction for GATA-1 recovery. PML4 promotes hemoglobin production, especially in β-estradiol–induced G1E-ER4 cells.
PML4 promotes terminal erythroid differentiation through GATA-1. (A-C) Forced expression of PML4 promotes K562 erythroid differentiation. K562 cells were infected with a retrovirus that expressed PML4 cDNA. (A) Western blot was performed to detect overexpression of PML. (B) Real-time RT-PCR was performed for AHSP and globin gene expression. (C) Flow cytometry was used to detect expression of GPA. (D-F) PML knockdown in K562 cells leads to reduced expression of erythroid genes. K562 cells were infected with a retrovirus harboring an shRNA to knock down PML (or GFP as a control). (D) Western blot assay, (E) real-time RT-PCR, and (F) flow cytometry were performed as in A-C. (G) Human primary erythroid progenitors were expanded and induced to erythroid differentiation by EPO. At day 1 of differentiation, retroviral vectors for PML4 overexpression or PML knockdown were transfected. PML and β-like globin genes expression were determined on day 4 of differentiation. (H-J) PML4 promoted erythroid maturation of G1E-ER4 but not G1E. PML4 and control plasmids were stably overexpressed in G1E and G1E-ER4 cells by retrovirus infection and induced to erythroid differentiation by adding β-estradiol for 18 hours of treatment. (H) Western blotting analysis of GATA-1 and PML4 expression in PML4 overexpressing and control G1E, G1E-ER4 cells before and after β-estradiol induction. (I) Real-time RT-PCR analysis of AHSP and α- and β-globin genes expression in PML4 overexpressing and control G1E and G1E-ER4 cells before and after β-estradiol induction. Right panel shows the enlarged bars from G1E cells. (J) Benzidine staining of PML4 overexpressing and control G1E-ER4 cells before and after 18 hours of β-estradiol induction for GATA-1 recovery. PML4 promotes hemoglobin production, especially in β-estradiol–induced G1E-ER4 cells.
We next characterized the expression and influence of PML4 in primary erythroid cells derived from human cord blood CD34+ progenitors. After in vitro expansion of the progenitors, the cells were induced to erythroid differentiation with EPO, expression of PML isoforms 1 to 4 was checked during differentiation. PML4 expression increases in the first 7 days of terminal cell divisions but shows little difference to that of isoforms 1 to 3. On prolonged terminal erythroid differentiation to day 11, a specific increase of PML4 level is observed among all 4 PML isoforms (supplemental Figure 6). Retroviruses that either overexpress PML4 or knockdown PML were then transfected into the primary erythroid cells of terminal cell divisions to determine the role of PML in erythroid differentiation. Real-time RT-PCR analysis showed that expression of the globin genes increased on PML4 overexpression and was down-regulated on PML attenuation in human primary erythroid cells, similar to our observations in K562 cells (Figure 1G; supplemental Figures 4B and 7A). On the contrary, transient overexpression of PML4 in proliferating CD34+ progenitors results in a slight setback of erythroid markers in the subsequent erythroid differentiation of the cells and promotes the expression of a few leukocyte markers (supplemental Figure 7B).
The range and degree of the effect of PML4 on terminal erythroid differentiation genes resemble that of GATA-1 (supplemental Figure 8), and the GATA-1 pathway was affected in PML4-overexpressing K562 cells. To assess whether the role of PML4 in erythroid cells may be dependent on GATA-1, mouse GATA-1–null embryonic stem (G1E) cells and GATA-1 stably rescued G1E-ER4 cells were examined. PML4 was forcibly expressed in G1E and G1E-ER4 cells, respectively, followed by β-estradiol induction, which restores GATA-1 activity and erythroid differentiation in G1E-ER4 cells. Forced expression of PML and recovery of GATA-1 were confirmed by western blotting (Figure 1H), and expression of AHSP and the adult globin genes was readily detected. In G1E cells, neither adult globin gene nor AHSP expression was obviously affected by PML4 overexpression, either before or after β-estradiol addition, whereas in G1E-ER4 cells, the adult globin genes and the AHSP gene were activated on PML4 overexpression, and the enhancing effect became even more noticeable after β-estradiol treatment, which induces increased GATA-1 activity (Figure 1I). Benzidine staining further showed that PML4 increases hemoglobin production in G1E-ER4 cells, especially after β-estradiol–induced GATA-1 restoration (Figure 1J). Altogether, these observations support the notion that PML4 activity is, directly or indirectly, dependent on GATA-1 activity during erythroid differentiation.
GATA-1 specifically interacts with PML4
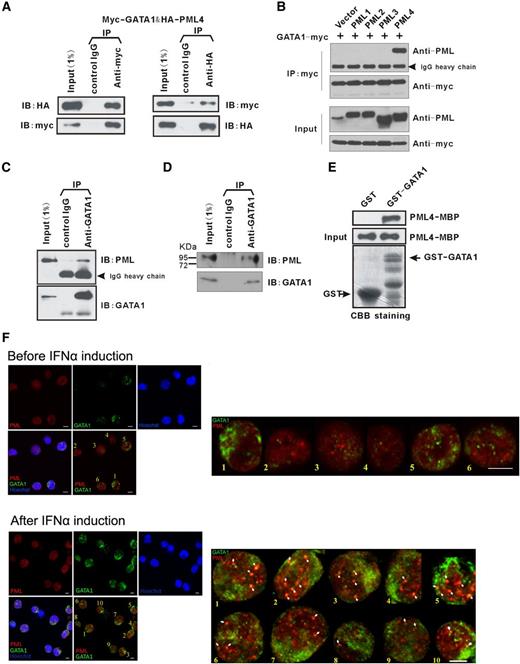
To elucidate how PML4 function in erythroid cells may involve GATA-1 participation, we first examined GATA-1 expression in PML4 overexpressed or knockdown K562 cells, but observed no obvious change (Figure 1A,D). We therefore next investigated whether PML4 physically interacts with GATA-1. Cotransfection of 293T cells with Myc-tagged GATA-1 and HA-tagged PML4, followed by bidirectional Co-IP assays with anti-tag antibodies, showed that PML4 and GATA-1 reciprocally immunoprecipitate, indicating physical interaction between PML4 and GATA-1 (Figure 2A). Other nuclear PML isoforms were also tested for their ability to interact with GATA-1. Among those PML isoforms, from 1 to 4, only PML4 was able to interact with GATA-1 under these experimental conditions, suggesting that the interaction with GATA-1 is PML4 isoform specific (Figure 2B). The interaction of PML4 with endogenous GATA-1 was confirmed in erythroid G1E-ER4 cells that stably expressed PML4 using GATA-1 and PML antibodies (Figure 2C). Finally, Co-IP assay in human umbilical HPC-derived primary erythroid cells detected a unique ∼95-kDa PML band using PG-M3 monoclonal antibody out of precipitates generated using the anti–GATA-1 antibody. Considering that the other PML isoforms of similar size, namely PML1-3, cannot interact with GATA-1 in vitro, this analysis indicated the interaction of endogenous PML4 and GATA-1 (Figure 2D).
PML4 directly interacts with GATA-1. (A) Reciprocal Co-IP of exogenous HA-PML4 and myc-GATA-1 in 293T. (B) Interaction of GATA-1 and PML isoforms in IP assays. GATA-1 interacts with PML4 specifically. (C) Interaction of PML4 and endogenous GATA-1 in β-estradiol–induced G1E-ER4 cells. IP was performed with rat anti–GATA-1(N6), followed by immunoblotting with anti-PML (PG-M3). (D) Interaction of endogenous PML and GATA-1 in erythroid-differentiated human umbilical CD34+ cells. (E) GST-pulldown assay with in vitro expressed GATA-1 (fused to GST) and PML4 (fused to MBP) shows direct interaction between PML4 and GATA-1. CBB staining, Coomassie brilliant blue staining. (F) Colocalization of endogenous PML and GATA-1 in erythroid-differentiated human umbilical cord CD34+ cells. Immunofluorescence analyses of endogenous PML (red) and GATA-1 (green) were carried out in the primary erythroid cells at day 6 of EPO induction without (upper) or with (lower) IFNα induction (1000 U/mL, 24 hours) of PML expression. The white arrows in the enlarged panels indicate sites of colocalization. All images were acquired on an Olympus FV1000 Confocal microscope using the Olympus FV1000 Viewer software, version 3.0a; scale bars, 5 μm.
PML4 directly interacts with GATA-1. (A) Reciprocal Co-IP of exogenous HA-PML4 and myc-GATA-1 in 293T. (B) Interaction of GATA-1 and PML isoforms in IP assays. GATA-1 interacts with PML4 specifically. (C) Interaction of PML4 and endogenous GATA-1 in β-estradiol–induced G1E-ER4 cells. IP was performed with rat anti–GATA-1(N6), followed by immunoblotting with anti-PML (PG-M3). (D) Interaction of endogenous PML and GATA-1 in erythroid-differentiated human umbilical CD34+ cells. (E) GST-pulldown assay with in vitro expressed GATA-1 (fused to GST) and PML4 (fused to MBP) shows direct interaction between PML4 and GATA-1. CBB staining, Coomassie brilliant blue staining. (F) Colocalization of endogenous PML and GATA-1 in erythroid-differentiated human umbilical cord CD34+ cells. Immunofluorescence analyses of endogenous PML (red) and GATA-1 (green) were carried out in the primary erythroid cells at day 6 of EPO induction without (upper) or with (lower) IFNα induction (1000 U/mL, 24 hours) of PML expression. The white arrows in the enlarged panels indicate sites of colocalization. All images were acquired on an Olympus FV1000 Confocal microscope using the Olympus FV1000 Viewer software, version 3.0a; scale bars, 5 μm.
To further address whether PML4 directly interacts with GATA-1, we performed GST pull-down assays with GST-tagged GATA-1 and MBP-tagged PML4. As shown in Figure 2E, PML4-MBP proteins bound to glutathione beads infused with the GATA1-GST fusion protein, but not with GST protein alone, indicating that PML4 interacted directly with GATA-1. Finally, immunofluorescence assays were used to examine the cellular localizations of GATA-1 and PML. The fluorescence signals of endogenous GATA-1 and PML partially overlapped in human umbilical CD34+-derived erythroid cells at day 6 of EPO induction, but GATA-1 was recruited into the PML nuclear bodies after IFNα induction of PML expression in these cells. Interestingly, increasing GATA-1:FOG1 colocalization was also observed after IFNα treatment (Figure 2F; supplemental Figure 9).
Coiled-coil domain of PML and C-terminal zinc finger of GATA-1 are important for the PML:GATA-1 interaction
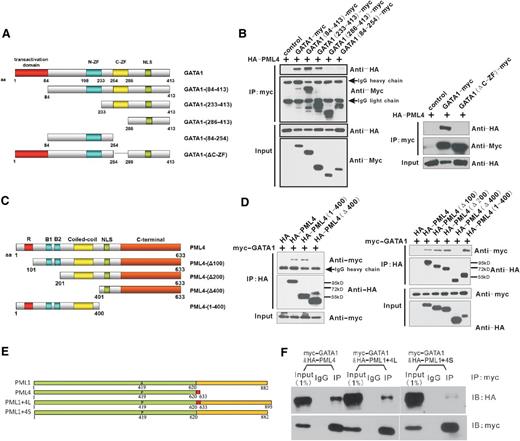
To map the domains that are critical for the physical interaction of PML4 and GATA-1, we constructed a series of truncated mutants of the 2 proteins (Figure 3A,C). GATA-1 contains a transcriptional activation domain in the N terminus and two zinc-finger domains in the middle.21 The N-terminal and C-terminal zinc fingers are required for binding to DNA and interaction with many known protein partners.22,23 An IP assay showed that PML4 does not interact with truncated mutants of GATA-1 that lack the C-terminal zinc finger (Figure 3B). Therefore, the C-terminal zinc finger of GATA-1 is crucial for the PML4:GATA-1 physical interaction.
The coiled-coil domain of PML and C-terminal zinc finger of GATA-1 mediate the PML:GATA-1 interaction. (A) Structure of full-length and truncated GATA-1 fragments used in B. GATA-1–expressing plasmids were sequentially truncated to produce the GATA1-(84-413), GATA1-(233-413), GATA1-(286-413), or GATA1-(84-245) or mutated to produce GATA1-(ΔC-ZF) that specifically deleted the C-terminal zinc finger for subsequent analysis of the critical domains for the GATA1:PML4 interaction. (B) Co-IP assay of differentially truncated/mutated GATA-1 fragments and full-length PML4. All GATA-1 truncates/mutates without the C-terminal zinc finger lost the ability to interact with PML4, indicating that the C-terminal zinc finger is essential for interaction with PML4. (C) Structure of full-length and truncated PML4 fragments used in D. PML4-expressing plasmids were sequentially truncated from the N-terminal to produce the PML4-(Δ100), PML4-(Δ200), and PML4-(Δ400) that lost the first 100, 200, and 400 aa or the PML4-(1-400) that deleted the C-terminal domain. (D) Co-IP assay of different PML4 truncates and full-length GATA-1. Deletion of the N-terminal 400 aa, as in PML4-(Δ400), impairs GATA-1–interacting ability of PML4, while truncation of the first 100 or 200 aa, as in PML4-(Δ100) or PML4-(Δ200), shows nonaffected or even increased GATA-1 interaction, indicating that the coiled-coil domain located in the 200 to 400 aa of PML is indispensable for the interaction with GATA-1. (E) The structure of 2 chimeric molecules generated by fusing C-terminal domain of PML1 (621-882 aa) to full-length PML4 (produces the PML1+4L) or to the 1 to 620 aa of PML4 (produces the PML1+4S). (F) Co-IP assay of the interaction between GATA-1 and the chimeric molecules PML1+4L and PML1+4S, showing that C-terminal PML1 partially disrupts the PML4:GATA-1 interaction, whereas the 13 aa at C-terminal PML4 (621-633 aa) is important for maintaining the GATA-1 interaction.
The coiled-coil domain of PML and C-terminal zinc finger of GATA-1 mediate the PML:GATA-1 interaction. (A) Structure of full-length and truncated GATA-1 fragments used in B. GATA-1–expressing plasmids were sequentially truncated to produce the GATA1-(84-413), GATA1-(233-413), GATA1-(286-413), or GATA1-(84-245) or mutated to produce GATA1-(ΔC-ZF) that specifically deleted the C-terminal zinc finger for subsequent analysis of the critical domains for the GATA1:PML4 interaction. (B) Co-IP assay of differentially truncated/mutated GATA-1 fragments and full-length PML4. All GATA-1 truncates/mutates without the C-terminal zinc finger lost the ability to interact with PML4, indicating that the C-terminal zinc finger is essential for interaction with PML4. (C) Structure of full-length and truncated PML4 fragments used in D. PML4-expressing plasmids were sequentially truncated from the N-terminal to produce the PML4-(Δ100), PML4-(Δ200), and PML4-(Δ400) that lost the first 100, 200, and 400 aa or the PML4-(1-400) that deleted the C-terminal domain. (D) Co-IP assay of different PML4 truncates and full-length GATA-1. Deletion of the N-terminal 400 aa, as in PML4-(Δ400), impairs GATA-1–interacting ability of PML4, while truncation of the first 100 or 200 aa, as in PML4-(Δ100) or PML4-(Δ200), shows nonaffected or even increased GATA-1 interaction, indicating that the coiled-coil domain located in the 200 to 400 aa of PML is indispensable for the interaction with GATA-1. (E) The structure of 2 chimeric molecules generated by fusing C-terminal domain of PML1 (621-882 aa) to full-length PML4 (produces the PML1+4L) or to the 1 to 620 aa of PML4 (produces the PML1+4S). (F) Co-IP assay of the interaction between GATA-1 and the chimeric molecules PML1+4L and PML1+4S, showing that C-terminal PML1 partially disrupts the PML4:GATA-1 interaction, whereas the 13 aa at C-terminal PML4 (621-633 aa) is important for maintaining the GATA-1 interaction.
All PML isoforms share in common an RBCC motif in their N-terminal regions, which is essential for PML nuclear body formation in vivo and mediates multiple protein-protein interactions.12 IP assays showed that the RBCC motif–containing N-terminal region of PML (amino acids [aa] 1-400) is necessary for the PML4:GATA-1 interaction. More refined truncations pinpointed the coiled-coil domain as the essential domain of PML4 for the GATA-1 interaction (Figure 3D). Overall, these results revealed that the carboxy zinc finger of GATA-1 and the coiled-coil domain of PML4 mediate the direct interaction between GATA-1 and PML4.
To further investigate the isoform specificity of the PML and GATA-1 interaction, 2 chimeric molecules, PML1+4L and PML1+4S, were generated by fusing C-terminal 621 to 882 aa of PML1 to full-length PML4 or to the 1 to 620 aa of PML4. Subsequent Co-IP analysis of the interaction between GATA-1 and the chimeric molecules showed that C-terminal of PML1 partially disrupts the PML:GATA-1 interaction, whereas the 13 aa C-terminal of PML4 is important for maintaining the GATA-1 interaction (Figure 3E-F), indicating that both domains contribute to the isoform specificity of the PML:GATA-1 interaction.
PML4 enhances the trans-activating activity of GATA-1
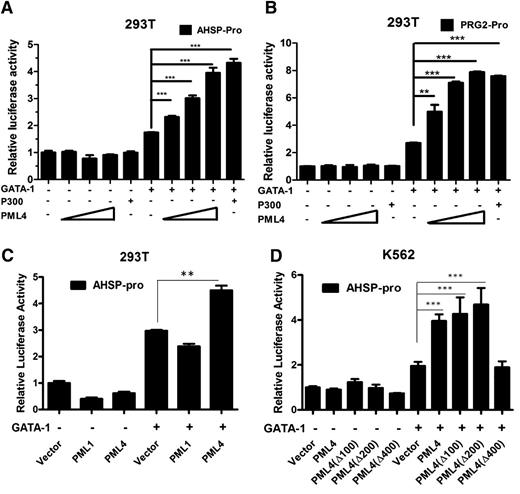
PML interacts with and modulates the activities of many transcriptional factors, including p53, myc, and NFAT.14,24,25 Therefore, we next tested whether PML might also affect the trans-activation activity of GATA-1. Two luciferase reporters, containing either the promoter region of AHSP or that of proteoglycan 2 gene (PRG2),26 were constructed. Both AHSP and PRG2 represent typical GATA-1 target genes. The reporter constructs were cotransfected into 293T cells together with GATA-1 and increasing amounts of PML4-expressing plasmids. The results showed that PML4 enhances GATA-1–mediated reporter activity in a dose-dependent manner with both reporter genes used in the assay, whereas it exhibits no regulatory effect in the absence of cotransfected GATA-1 (Figure 4A-B). Having shown that GATA-1 interacts specifically with the PML4 isoform, we then examined the possible influence of PML1, the longest PML isoform, on GATA-1–mediated transcriptional activation. In contrast to the positive influence of PML4, PML1 could not stimulate GATA-1 transcriptional activity (Figure 4C).
Transcriptional activity of GATA-1 is enhanced by PML4. (A-B) PML4 enhanced GATA-1 transactivity in a luciferase reporter assay. 293T cells were cotransfected with (A) pro-AHSP-pGL3-basic (AHSP-pro) or (B) pro-PRG2- pGL3-basic (PRG2-pro) and pRL-TK in the presence or absence of GATA-1 and increased gradient of PML4 expression vectors. Transfected cells were cultured for 24 hours and lysed for measurement of luciferase activities. p300 was used as the positive control in the reporter assays. (C) PML1 did not increase GATA-1 transactivity as PML4 did. 293T cells were transfected with AHSP-pro, pRL-TK, PML1, or PML4 with or without GATA-1 as indicated. (D) Influence of truncated PML4 fragments on transactivation ability of GATA-1. The Δ400 PML mutant that cannot interact with GATA-1 also shows loss of cooperation with GATA-1. All data are representative of 3 independent experiments. Error bars represent standard error of the mean.
Transcriptional activity of GATA-1 is enhanced by PML4. (A-B) PML4 enhanced GATA-1 transactivity in a luciferase reporter assay. 293T cells were cotransfected with (A) pro-AHSP-pGL3-basic (AHSP-pro) or (B) pro-PRG2- pGL3-basic (PRG2-pro) and pRL-TK in the presence or absence of GATA-1 and increased gradient of PML4 expression vectors. Transfected cells were cultured for 24 hours and lysed for measurement of luciferase activities. p300 was used as the positive control in the reporter assays. (C) PML1 did not increase GATA-1 transactivity as PML4 did. 293T cells were transfected with AHSP-pro, pRL-TK, PML1, or PML4 with or without GATA-1 as indicated. (D) Influence of truncated PML4 fragments on transactivation ability of GATA-1. The Δ400 PML mutant that cannot interact with GATA-1 also shows loss of cooperation with GATA-1. All data are representative of 3 independent experiments. Error bars represent standard error of the mean.
To further investigate whether the PML4:GATA-1 interaction is essential for PML4 influence on GATA-1 activity, reporter assays were performed using variously truncated PML4 in vitro–created isoforms. These results showed that deletion of the coiled-coil domain found to be essential for the PML4:GATA-1 interaction reduced PML4 enhancement on GATA-1 activity to background levels (Figure 4D). These results support the notion that a direct physical interaction between these 2 proteins mediates their functional cooperation.
PML4 stimulates GATA-1-p300 cooperation and acetylation of GATA-1
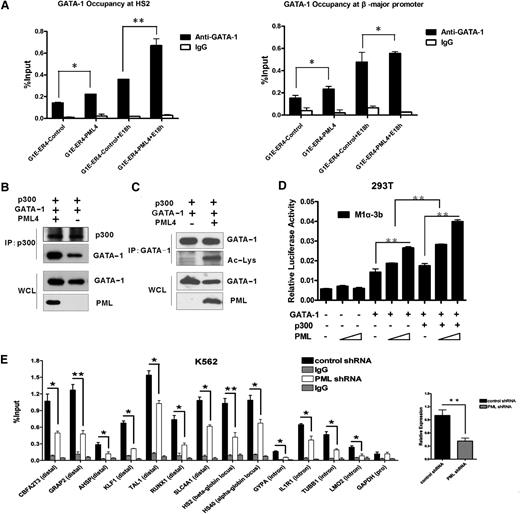
To further analyze how PML might influence GATA-1 activity and perhaps reveal the molecular mechanisms underlying PML-mediated promotion of erythroid differentiation, we next performed electrophoretic mobility shift assays to detect any influence of PML on GATA-1 DNA binding ability in vitro. A biotin-labeled DNA probe bearing the core canonical GATA DNA sequence27 was incubated with nuclear extracts prepared from GATA-1–transfected 293A cells, and a specific shift of the DNA-protein complex was generated only after GATA-1 transfection, indicating that GATA-1 efficiently bound to this probe. However, when PML4 was cotransfected with GATA-1, there was no obvious change in the band shift intensity (supplemental Figure 10). Transfection with increasing amounts of PML4 also did not affect band shift intensity (supplemental Figure 10). To assess whether PML affects the chromatin occupancy of GATA-1 in erythroid cells in vivo, we monitored GATA-1 recruitment to HS2 and to the β-major gene within the β-globin locus after PML4 overexpression in G1E-ER4 cells, either before or after β-estradiol induction, and found that PML4 forced expression increased GATA-1 chromatin occupancy at both sites (Figure 5A). These results demonstrated that PML4 enhances the DNA binding ability of GATA-1 in vivo but not in vitro.
PML4 stimulates cooperation of GATA-1 and p300. (A) PML4 increased in vivo DNA binding ability of GATA-1. ChIP analysis was performed to detect GATA-1 occupancy at HS2 and β-major promoter in G1E-ER4 cells expressing PML4 and vector control before and after 18-hour β-estradiol induction for GATA-1 activity recovery (*P < .05, **P < .01). (B-C) 293T cells were transfected with p300 and GATA-1 expression vectors in the presence or absence of PML4 expression vector. Cell lysates were prepared and immunoprecipitated by anti-p300 (B) or anti–GATA-1 (C), followed by immunoblotting with indicated antibodies. (D) PML4 enhanced cooperation of GATA-1 and p300. 293T cells were cotransfected with indicated reporter constructs and pRL-TK in the presence or absence of expression vectors for GATA-1, PML4, and p300. Transfected cells were cultured for 24 hours and lysed for measurement of luciferase activities. Data are representative of 3 independent experiments. Error bars represent standard error of the mean. (E) Occupancy of p300 at GATA-1 binding sites in PML knockdown and control K562 cells. GATA-1 binding sites at distal regions or within intron sequences of GATA-1 target genes (as indicated) were detected. glyceraldehyde 3-phosphaate dehydrogenase–promoter region was used as negative control. Right panel shows the efficiency of PML interference. Primer sequences are available on request.
PML4 stimulates cooperation of GATA-1 and p300. (A) PML4 increased in vivo DNA binding ability of GATA-1. ChIP analysis was performed to detect GATA-1 occupancy at HS2 and β-major promoter in G1E-ER4 cells expressing PML4 and vector control before and after 18-hour β-estradiol induction for GATA-1 activity recovery (*P < .05, **P < .01). (B-C) 293T cells were transfected with p300 and GATA-1 expression vectors in the presence or absence of PML4 expression vector. Cell lysates were prepared and immunoprecipitated by anti-p300 (B) or anti–GATA-1 (C), followed by immunoblotting with indicated antibodies. (D) PML4 enhanced cooperation of GATA-1 and p300. 293T cells were cotransfected with indicated reporter constructs and pRL-TK in the presence or absence of expression vectors for GATA-1, PML4, and p300. Transfected cells were cultured for 24 hours and lysed for measurement of luciferase activities. Data are representative of 3 independent experiments. Error bars represent standard error of the mean. (E) Occupancy of p300 at GATA-1 binding sites in PML knockdown and control K562 cells. GATA-1 binding sites at distal regions or within intron sequences of GATA-1 target genes (as indicated) were detected. glyceraldehyde 3-phosphaate dehydrogenase–promoter region was used as negative control. Right panel shows the efficiency of PML interference. Primer sequences are available on request.
To further investigate this curious observation, we next examined whether PML4 might affect cooperation of GATA-1 with its known array of coactivating cofactors. Using Co-IP analysis, we discovered that PML4 promoted the interaction of GATA-1 with p300 (Figure 5B). Because GATA-1 is acetylated by p300 in vivo and in vitro,4,5 we compared acetyl GATA-1 levels before and after PML4 overexpression. The results of this experiment demonstrated that PML4 indeed increases the acetylation of GATA-1 (Figure 5C). The observation that PML4 enhances GATA-1 acetylation and in vivo DNA binding is consistent with a previous report demonstrating that mutation of acetylation sites in GATA-1 selectively impairs the chromatin occupancy of GATA-1 at its binding sites in vivo while exerting no effect on in vitro DNA binding,28 a phenomenon that has recently been attributed to the binding of Brd3.29 Luciferase reporter assays further showed that the stimulatory effect of PML4 on GATA-1 trans-activation activity was strongly enhanced when p300 was cotransfected (Figure 5D), indicating that PML4 facilitates the functional cooperation of GATA-1 with its cofactor p300. In addition, ChIP assays demonstrated that the in vivo occupancy of p300 at GATA-1 binding sites was reduced on PML knockdown in K562 cells (Figure 5E). Taken together, these results demonstrate that PML4 facilitates cooperation between GATA-1 and p300 to promote the DNA binding and transcriptional activity of GATA-1 in vivo.
Discussion
GATA-1 is a critical transcriptional regulator of erythroid differentiation whose transcriptional activity remains under intensive investigation. Here, we demonstrate that PML4 specifically promotes erythroid gene expression and erythroid differentiation in a GATA-1–dependent fashion. We show that PML4 exerts this effect by interacting directly with GATA-1 to enhance its transcriptional activity, which, at least in part, can be attributed to the stimulation of GATA-1/p300 functional cooperation and GATA-1 acetylation. These studies indicate the important role played by PML in erythroid differentiation and immediately identify PML4 as a new potential target for directed therapy of GATA-1–related diseases.
During erythroid differentiation, the expression profiles of GATA-1 and GATA-2 exhibit a unique switching mode. Expression of GATA-2 is limited to early hematopoietic progenitors, whereas GATA-1 is typically present in more mature erythroid cells. PML was reported to be able to bind GATA-2 and regulate its activity, and it was suggested that this interaction could preferentially contribute to the pathogenesis of PML/retinoic acid receptor leukemic cells.30 We found herein that PML4 directly interacts with GATA-1 through its coiled-coil domain. PML modulates the trans-activating activities of many transcriptional factors to which it binds. We demonstrated here that GATA-1 can also be recruited into PML bodies and that GATA-1 trans-activation activity is enhanced in the presence of PML4 in a dose-dependent manner. Flow cytometric analysis shows significant increase in the ratio of GPA-positive cells in K562 cells in which PML4 is forcibly expressed, an observation that is consistent with a previous report that PML inhibition results in repressed erythroid colony formation from hematopoietic progenitors. In addition, we show that PML4 facilitates endogenous globin and AHSP gene expression in K562 cells, in primary erythrocytes, and in GATA-1–rescued G1E-ER4 cells, but not in GATA-1–deficient G1E cells. These observations support the notion of a pleiotropic role for PML in both erythroid differentiation and maturation and are consistent with the central role GATA-1 known to play in erythropoiesis.
Unlike GATA-1–null mutant mice, PML knockout mice have not been reported to have obvious anemia. Considering that a small number of GATA-1 knockdown mice expressing ∼20% of wild-type GATA-1 levels survive to adulthood,31 diminished GATA-1 activity in PML−/− mice may be tolerated or may be compensated by extrinsic erythroid-stimulating signals such as EPO. A functional discrepancy between human and mouse PML isoforms, which has been shown to result in differential regulation of MHC class I expression between the 2 species, might also account for the aphenotypic erythropoiesis in PML−/− mice.19 The GATA-1.05 erythroid cell was found to be tumorigenic because the trace mount of GATA-1 expressed in these cells (5%) is sufficient to inhibit apoptosis but is insufficient to suppress erythroid progenitor proliferation and to promote terminal erythroid differentiation.32 PML may therefore provide a potential target for treating a subset of GATA-1–related diseases by fine tuning GATA-1 activity. Besides affecting erythroid differentiation, the combinatorial effect of PML4 and GATA-1 may also contribute to other cellular processes, such as megakaryotic differentiation where GATA-1 activity is equally essential.32
Specific functions have been assigned to individual PML isoforms. PML1 specifically interacts with AML1 to promote transcriptional enhancement and stimulate myeloid differentiation,33 PML3 plays a direct role in centrosome duplication through the suppression of Aurora A kinase activation34 and is involved in the cell proliferation/differentiation decision by interacting with pRb. PML4 is involved in multiple physiological events, including senescence and granulocyte differentiation, through its regulatory interactions with p53 and c-myc, among others.14,35 Recent studies also indicate that PML4 affects E2 promoter-binding factor-related cell senescence36 and specifically regulates telomerase activity by directly and negatively regulating telomerase reverse transcriptase activity.37
The present study revealed that PML4 specifically interacts with GATA-1. Although all PML isoforms share a coiled-coil domain that is important for the GATA-1 interaction, and the PML-(1-400) that is entirely devoid of the C-terminal domain interacts with GATA-1, we show that the GATA-1 interaction is restricted to PML4 among all the 4 isoforms analyzed and that the C-terminal domain of PML is actively involved in regulating their interaction to GATA-1. Functional analysis showed that PML4, but not the GATA-1–defective PML4(Δ400) or PML1, synergizes with GATA-1 in stimulating erythroid gene expression, supporting the hypothesis that there exists functional specificity of PML4 in regulating erythropoiesis. How the different PML isoforms differ in functional specificity because of subtle differences in their C-terminal domains and the coordination between different isoforms remain intriguing issues to be investigated.
Differing from general cofactors like histone modifiers and chromatin remodeling enzymes, PML has no intrinsic enzymatic activity. It has been reported that PML enhances the activities of transcriptional factors p53, AML1, and NFAT by facilitating their colocalization and cooperation with CBP/p300.10,25,33 Consistent with this hypothesis, we demonstrate here that PML recruits GATA-1 into PML nuclear bodies, promotes cooperative transcriptional activation between GATA-1 and p300, and increases GATA-1 acetylation, in vivo DNA binding, and trans-activation activity. Unlike the in situ PML/special AT-rich sequence-binding protein 1 interaction at matrix attachment regions in regulating the MHC-I locus,38 no binding of PML was detected in vivo at GATA-1 sites, suggesting that the effect of PML on GATA-1 activity happens before GATA-1 targeting to downstream genes.
In addition to acetylation, GATA-1 and its coregulator FOG are both targets of sumoylation,39,40 implying additional layers of complexity in GATA-1 regulation by small ubiquitin-related modifier-1–enriched PML nuclear bodies.41 Recently, GATA-1 was shown to associate with 2 other PML interacting factors, p53 and pRb, in regulating erythroid cell cycle progression and apoptosis, 2 processes that are intimately linked to erythropoiesis.42,43 Our work, together with the demonstrated functional interactions between GATA-1 and pRb, suggests that the coordinate effects of pRb and PML in the GATA1 pathway may underlie the reported synergy of these 2 factors in regulating erythropoiesis.15 Although the dynamics of these multiple complex interactions await further detailed characterization, these observations suggest that PML nuclear bodies function as a platform for efficient association of multiple interacting transcriptional factors and cofactors to provide coordinated regulation to erythropoiesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Professor Mitchell J. Weiss for kindly providing the G1E/G1E-ER4 cells, Professor Kun-Sang Chang for kindly providing the PML plasmids and anti-PML antibody, and Professor James Douglas Engel for his kind help in language editing and in the revision of the manuscript. The authors also thank Professor Mao-Jun Yang for suggestions in designing the chimeric PML constructs.
This work was supported by National Natural Science Foundation of China grant 31030026, 973 Program grants 2011CB965203, 2011CB964803, and 2010CB529903, 863 Program grant 2011AA020116, and National Natural Science Foundation of China grant 31021091.
Authorship
Contribution: J.W. and L.-Q.Z. performed most of the experiments and participated in data interpretation and manuscript preparation; W.Y., X.-M.X., W.-T.W., and Z.X. participated in umbilical cord blood isolation, primary erythroid cell culture, and retroviral preparation; W.Y., Z.-G.Z., J.X., M.L., X.W., P.Z., and B.-B.M. participated in plasmid construction, reporter assay, immunobloting, ChIP, real-time RT-PCR analyses, and the immunofluorescence assay; D.-L.H. assisted in cell culture; and X.L. and D.-P.L. conceived the study, carried out the experimental design and data interpretation, and prepared and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: De-Pei Liu, Peking Union Medical College, 5 Dong Dan San Tiao, Beijing, 100005 Republic of China; e-mail: liudp@pumc.edu.cn; and Xiang Lv, Peking Union Medical College, 5 Dong Dan San Tiao, Beijing, 100005 Republic of China; e-mail: lvxiang@pumc.edu.cn.
References
Author notes
J.W., L.-Q.Z., and W.Y. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal