In this issue of Blood, Nelson et al1 describe a novel somatic ARAF mutation in a child with Langerhans cell histiocytosis (LCH) and demonstrate that the encoded protein has strong gain-of-function properties. Importantly, this mutant A-Raf molecule is sensitive to inhibition by vemurafenib, a potent and selective Raf kinase inhibitor that is Food and Drug Administration (FDA)-approved for the treatment of advanced melanoma.2,3 This work thus identifies a new driver mutation in LCH that is potentially actionable in the clinic.
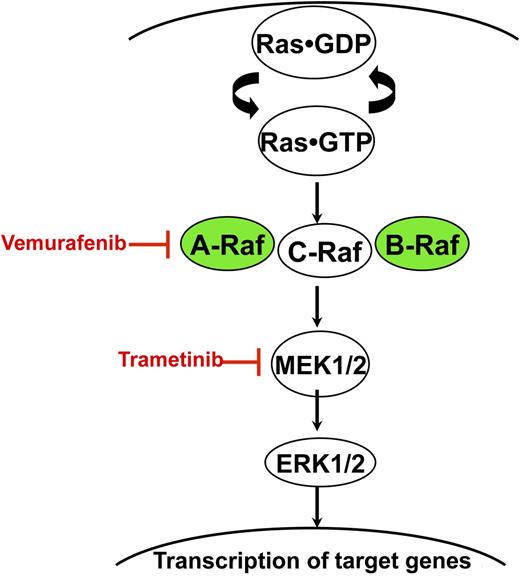
Ras accumulates in the active GTP-bound conformation in response to growth factor stimulation and other extracellular stimuli. Normal Raf proteins bind to Ras-GTP and signal as homo- and heterodimers. BRAF and ARAF mutations found in LCH and other cancers exhibit constitutive kinase activation, which results in the sequential activation of MEK and ERK. Vemurafenib and trametinib are potent and selective inhibitors of Raf and MEK, respectively.
Ras accumulates in the active GTP-bound conformation in response to growth factor stimulation and other extracellular stimuli. Normal Raf proteins bind to Ras-GTP and signal as homo- and heterodimers. BRAF and ARAF mutations found in LCH and other cancers exhibit constitutive kinase activation, which results in the sequential activation of MEK and ERK. Vemurafenib and trametinib are potent and selective inhibitors of Raf and MEK, respectively.
LCH is a rare hematologic disorder that is classified as a unified disease entity based on common histopathologic features and the proliferation of cells with phenotypic and cell surface marker expression characteristic of Langerhans cells.4 However, the clinical presentation is highly variable, ranging from generally benign single system lesions to life-threatening multisystem disease with organ dysfunction. The antigen-presenting function of Langerhan’s cells, mixed population of infiltrating leukocytes in pathologic specimens, and protean clinical manifestations naturally raised questions regarding whether LCH is primarily an immunologic or neoplastic disease. Studies demonstrating that LCH lesions are clonal followed by genetic analysis demonstrating somatic BRAFV600E mutations in >50% of cases provided compelling evidence that LCH is a hematologic neoplasm.4,5 Although BRAFV600E mutations do not define clinical risk groups,5,6 they are associated with a higher incidence of disease recurrence. Interestingly, Beres et al6 recently reported that BRAFV600E mutations are restricted to lesional CD207+ cells in low-risk patients. However, high-risk patients carried BRAFV600E mutations in CD207+ lesional cells, circulating CD11c+ and CD14+ cells and bone marrow CD34+ hematopoietic cell progenitors. These data suggest that high-risk LCH arises from somatic mutation of hematopoietic stem/progenitor cells, whereas low-risk LCH arises in tissue-restricted precursor dendritic cells.6 In addition to advancing our understanding of LCH pathogenesis, the presence of BRAFV600E mutations in many patient specimens has immediate therapeutic implications, as the high prevalence of this mutation in melanoma and other cancers stimulated the development of small molecule inhibitors that induced genotype-specific responses in clinical trials.2,3,7
The report of Nelson et al adds a new piece to the LCH puzzle. The current work was stimulated by the provocative observation that LCH lesions with and without BRAF mutations exhibit high levels of phosphorylated extracellular signal-regulated kinase (ERK), an important effector kinase of Raf in the Ras/Raf/mitogen-activated protein kinase kinase (MEK)/ERK signaling cascade5 (see figure). This suggests that mutations in other genes cause ERK activation in LCH patients lacking BRAF mutations. The authors address this question by performing whole exome sequencing of DNA isolated from the LCH lesions and normal tissues of 3 children. They uncovered a BRAFV600E mutation in 1 case and, unexpectedly, a mutation in the related ARAF gene in lesional DNA from a second patient. Importantly, the BRAF and ARAF mutant allele frequencies were high in each case (45% and 63%, respectively), suggesting a growth advantage for mutant cells. Whereas ARAF mutations were thought to be uncommon in human cancer, a recent paper suggested that it is an oncogenic driver in ∼1% of lung adenocarcinomas.8 Given the potential significance of ARAF as a bona fide LCH oncogene and the complex mutation detected in this case (the mutant allele contains a sequence alteration that results in an amino acid substitution at codon 351 [F351L] and a 6-nucleotide in-frame deletion that removes amino acids 347 and 348), Nelson et al perform elegant functional analyses in which they demonstrate that the mutant A-Raf protein aberrantly activates recombinant MEK, a direct substrate of activated Raf (see figure). They further showed that the relative kinase activity of this mutant A-Raf molecule is comparable to B-RafV600E and that it can transform fibroblasts in a classic 3T3 soft agar assay. Finally and importantly, the authors show that this constitutively activated mutant A-Raf kinase is sensitive to inhibition by vemurafenib.
The identification and functional characterization of this novel ARAF mutation has biological and clinical implications. First, the discovery of a new somatic mutation altering the Ras/Raf/MEK/ERK signaling cascade reinforces the central role of this pathway in LCH pathogenesis and provides further impetus for comprehensive genomic analysis of additional cases lacking BRAF mutations. The unusual nature of the ARAF mutation described by Nelson et al suggested that it would be uncommon. Indeed, sequencing 23 other LCH specimens with normal BRAF failed to uncover additional ARAF mutations. Performing whole exome sequencing of additional patients without RAF gene mutations is a logical next step toward unraveling the pathogenesis of LCH. Second, this study has implications for establishing a diagnosis of LCH and for identifying patients who might benefit from targeted therapies. Although ARAF mutations appear to be uncommon in LCH, they are straightforward to test for and are clinically actionable. As mutations in other genes are identified and functionally validated, it should be feasible to develop a targeted molecular diagnostics panel for LCH that includes BRAF, ARAF, and other driver genes. Finally, this new study raises questions regarding optimal approaches for implementing pathway-directed treatments for LCH. In addition to vemurafenib, the FDA-approved MEK inhibitor trametinib7 is a rational therapeutic strategy for patients with LCH with mutations that aberrantly activate Raf/MEK/ERK signaling (see figure). However, because the long-term risks and benefits of these agents are unknown and other effective treatments exist for many patients with LCH, the optimal indications for administering a tyrosine kinase inhibitor—particularly to children—is an open question. A clinical trial of trametinib that is expected to open later this year in juvenile myelomonocytic leukemia, an aggressive myeloproliferative neoplasm of young children characterized by hyperactive Ras/Raf/MEK/ERK signaling,9 will generate safety data that might be relevant for pediatric patients with LCH. A recent report demonstrating rapid and dramatic responses of 3 adults with Erdheim-Chester disease or LCH with BRAFV600E mutations to vemurafenib underscores the clinical potential of targeted therapies for aggressive or refractory disease.10 Whereas patients with melanoma and other advanced cancers with oncogenic BRAF mutations almost invariably relapse after tyrosine kinase inhibitor therapy,2,3,7 durable responses seem more likely in LCH and other neoplasms characterized by extensive differentiation and limited clonal heterogeneity. Treating carefully selected patients and monitoring their clinical and molecular responses are required to test this hypothesis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal