In this issue of Blood, Flinn et al, Kahl et al, and Brown et al provide further encouragement that the possibility of a chemotherapy-free world is, indeed, a rapidly approaching reality in indolent non-Hodgkin lymphomas (NHLs), mantle cell lymphoma (MCL), and chronic lymphocytic leukemia (CLL).1-3
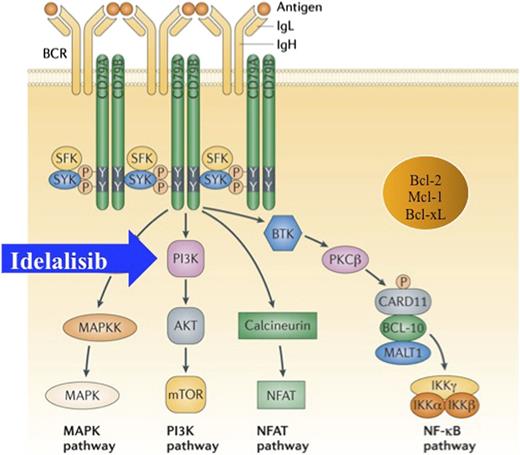
Chronic activation of the BCR engages multiple intracellular pathways. PI3K is inhibited by idelalisib. Bcl, B-cell lymphoma; Bcl-2, Bcl-2 gene; Bcl-xL, Bcl extra large; BCR, B-cell receptor; BTK, Bruton tyrosine kinase; CARD11, caspase recruiting domaine-11; IgL, immunoglobulin light chain; IKKa, inhibitor of NF-κB kinase; MALT1, mucosa-associated lymphoid tissue translocation protein 1; MAPK, mitogen-activated protein kinase; MAPKK, MAPK kinase; Mcl-1, myeloid cell leukemia differentiation protein-1; NfκB, nuclear factor κB; NFAT, nuclear factor of activated T cells; P, phosphorylation; PKCb, protein kinase C beta; SFK, SRC family kinase; SYK, spleen tyrosine kinase; Y, tyrosine. Figure is reproduced from Figure 2 in Young and Staudt11 with permission.
Chronic activation of the BCR engages multiple intracellular pathways. PI3K is inhibited by idelalisib. Bcl, B-cell lymphoma; Bcl-2, Bcl-2 gene; Bcl-xL, Bcl extra large; BCR, B-cell receptor; BTK, Bruton tyrosine kinase; CARD11, caspase recruiting domaine-11; IgL, immunoglobulin light chain; IKKa, inhibitor of NF-κB kinase; MALT1, mucosa-associated lymphoid tissue translocation protein 1; MAPK, mitogen-activated protein kinase; MAPKK, MAPK kinase; Mcl-1, myeloid cell leukemia differentiation protein-1; NfκB, nuclear factor κB; NFAT, nuclear factor of activated T cells; P, phosphorylation; PKCb, protein kinase C beta; SFK, SRC family kinase; SYK, spleen tyrosine kinase; Y, tyrosine. Figure is reproduced from Figure 2 in Young and Staudt11 with permission.
“The times they are a changin’”—Bob Dylan
The therapeutic holy grail for lymphoid malignancies has long been an effective chemotherapy-free strategy to avoid the scourge of the nonspecific toxic drugs that patients have been subjected to for decades, such as anthracyclines, alkylating agents, vinca alkyloids, and purine analogs. Initial forays into the nonchemotherapy realm included single-agent rituximab,4 doublets of monoclonal antibodies,5,6 or rituximab combined with the immunomodulatory agent lenalidomide.7 Each of these well-tolerated regimens produced high response rates with durable remissions. Nevertheless, the diseases remain incurable. Recent interest has focused on a series of agents that inhibit intracellular pathways that promote the proliferation and survival of malignant lymphocytes. Several of these pathways are activated through B-cell receptor signaling (see figure). One of the first such drugs was fostamatinib disodium, a spleen tyrosine kinase inhibitor that exhibited only modest activity and is not being actively pursued in lymphoid malignancies.8
More recently, idelalisib (formerly known as CAL-101 and GS-1101) has induced considerable interest. This potent, highly selective, orally bioavailable small molecule inhibits the δ isoform of phosphatidylinositol 3-kinase (PI3K), a pathway that is overactive in a number of lymphoid malignancies as well as solid tumors. PI3K exists in 4 different isoforms: p110α, -β, -γ, and -δ, the last being most relevant to B lymphocytes. As described in each of the 3 articles, PI3K-mediated phosphorylation activates the serine/threonine kinase AKT and, subsequently, mammalian target of rapamycin (mTOR). Overexpression of PI3K/AKT appears to contribute to the pathogenesis of various lymphoid malignancies, including indolent NHL, MCL, and CLL. Inhibiting PI3K results in cell death through apoptosis.
Rarely have we encountered enthusiasm for drugs such as that for idelalisib, which is based on phase 1 data. Flinn et al1 administered idelalisib to 64 heavily pretreated patients with indolent NHL, more than half of whom were refractory to previous chemotherapy. The response rate was 47%, but it was 69% in those treated at the now accepted dose of ≥150 mg twice daily, with a median progression-free survival (PFS) of 16.8 months at this dose level. These data have been confirmed in a subsequent phase 2 trial.9 Kahl et al2 treated 40 patients with MCL, with a response rate of 69% at a dose of ≥150 mg twice daily, with a PFS of 3.7 months that correlated with the extent of prior treatment. Brown et al3 reported on 54 patients with heavily pretreated, relapsed, or refractory CLL, many with adverse features. They demonstrated that idelalisib inhibited PI3Kδ, impairing Akt phosphorylation in patient CLL cells, significantly reducing serum levels of CLL-related chemokines and cytokines. When administered twice daily, idelalisib induced responses in 72% of patients, including 53% of those with 17p- and/or tumor protein 53 gene mutation, generally considered among patients with the most unfavorable prognoses. The overall median PFS was 32 months when using the currently recommended dose. Common to these 3 trials was a rapid tumor reduction with some responses lasting up to 2 years, and a favorable toxicity profile with the most prominent adverse effects being diarrhea, nausea, fever, fatigue, and an asymptomatic reversible transaminitis.
We are fortunate to have an increasing number of active oral drugs for patients with these diseases, including the Bruton tyrosine kinase inhibitor ibrutinib, the PI3K δ/γ inhibitor IPI-145, the BCL-2 inhibitor ABT-199, and those that target programmed death-1/programmed death ligand-1, among others.
However, despite the high response rates with each of them, most remissions are partial and, currently, these agents are administered as long as response and clinical and financial tolerance persist (they are expected to be quite unconscionably expensive). Those factors together have led to numerous trials to improve on their efficacy and limit the duration of treatment, such as combining them with other drugs, resulting in a better quality of response. A common backbone for ongoing clinical trials has been bendamustine plus rituximab. However, a more interesting avenue of investigation is combining them with other biologic agents. In a recent study, rituximab plus idelalisib achieved a survival benefit compared with rituximab alone in relapsed/refractory CLL patients with comorbidities.10 Other trials are exploring the additive benefit of these agents to the combination of rituximab-lenalidomide, such as those being piloted by the Alliance (formerly Cancer and Acute Leukemia Group B) with idelalisib in relapsed MCL or follicular lymphoma, and with ibrutininb as initial treatment of follicular lymphoma. Combinations of kinase inhibitors with each other or with proapoptotic agents are also of interest.
Ibrutinib is already approved by the US Food and Drug Administration for relapsed/refractory MCL and CLL and, hopefully, idelalisib will follow suit in short order. At that point, how do we decide which of these active agents to choose (if they are comparably priced)? Other than some differences in toxicity profile that may determine eligibility in a subset of patients (eg, coagulation abnormalities with ibrutinib, hepatic dysfunction with idelalisib), it may take comparative studies or biomarker assays to help us select appropriately. However, for now, we can provide our patients with new, effective, and less toxic treatment options for their diseases, and even more importantly, we can also provide hope that better treatments are still ahead. Although as single agents, these exciting drugs can transform indolent NHL, MCL, and CLL into more acceptable chronic disorders, it is quite possible that rational combinations will eventually lead to cure of these incurable diseases.
Conflict-of-interest disclosure: B.D.C. is a consultant for and has received research support from Gilead, Pharmacyclics/Janssen, and Roche-Genentech.