In this issue of Blood, Warkentin et al provide insight into certain rare but often catastrophic cases of thrombotic complications, termed “spontaneous” (or “autoimmune”) heparin-induced thrombocytopenia (HIT).1
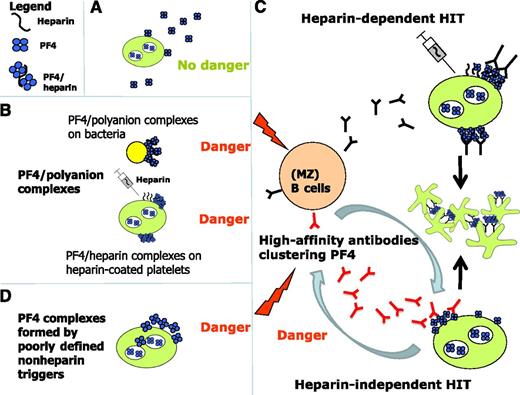
Mechanisms of induction of anti-PF4/polyanion antibodies. (A) Noncomplexed PF4 is not immunogenic. (B) However, when PF4 forms complexes with polyanions it undergoes a conformational change exposing epitopes to which PF4/heparin antibodies bind. These polyanions can be structures on bacterial surfaces or heparin bound to platelets. (C) The PF4/polyanion complexes induce antibody production of (most likely) MZ B cells (black antibodies, upper portion of panel C), which can induce platelet activation in the presence of heparin, that is, they are typically heparin-dependent. In delayed onset HIT and spontaneous HIT, the B cells produce antibodies which bind to, and potentially thereby cluster, PF4 and then activate platelets, typically in the absence of heparin (red antibodies, lower portion of panel C). It is unresolved which factors trigger these antibodies (D): potential candidates are PF4/bacteria clusters or PF4/nucleic acid clusters; it is also unclear whether heparin-independent platelet activation is a quantitative or a qualitative feature of these antibodies.
Mechanisms of induction of anti-PF4/polyanion antibodies. (A) Noncomplexed PF4 is not immunogenic. (B) However, when PF4 forms complexes with polyanions it undergoes a conformational change exposing epitopes to which PF4/heparin antibodies bind. These polyanions can be structures on bacterial surfaces or heparin bound to platelets. (C) The PF4/polyanion complexes induce antibody production of (most likely) MZ B cells (black antibodies, upper portion of panel C), which can induce platelet activation in the presence of heparin, that is, they are typically heparin-dependent. In delayed onset HIT and spontaneous HIT, the B cells produce antibodies which bind to, and potentially thereby cluster, PF4 and then activate platelets, typically in the absence of heparin (red antibodies, lower portion of panel C). It is unresolved which factors trigger these antibodies (D): potential candidates are PF4/bacteria clusters or PF4/nucleic acid clusters; it is also unclear whether heparin-independent platelet activation is a quantitative or a qualitative feature of these antibodies.
Some time ago, I consulted on the case of a 31-year-old woman admitted with severe headache and thrombocytopenia. Except for an upper respiratory tract infection that began 10 days earlier, she was healthy and taking no medications. Laboratory studies showed international normalized ratio 1.4, activated partial thromboplastin time 34 s, fibrinogen 0.6 g/L, d-dimer > 35 mg/L (<0.5), platelets 31 × 109/L, no bleeding, no signs of infection, and normal computed tomography (CT) head scan (to exclude sinus vein thrombosis). She received 4 g of fibrinogen and low molecular weight heparin (LMWH) for thrombosis prophylaxis. The next day, the platelet count was 15, she developed deep vein thrombosis, and her headache persisted. Although HIT seemed implausible, the coincidence of worsening thrombocytopenia and new thrombosis during LMWH prompted HIT testing, which showed, to my surprise, high-titer platelet-activating anti-PF4/heparin immunoglobulin G (IgG) antibodies. The functional heparin-induced platelet activation test (HIPA) was also strongly positive in the sample without addition of heparin. Even when testing was repeated using a pre-LMWH admission sample, the same result was obtained. Despite the immediate start of therapeutic-dose danaparoid anticoagulation, she deteriorated neurologically the same day, and massive sinus vein thrombosis associated with intracerebral bleeding was demonstrated by repeat CT imaging. Despite eventual platelet count normalization over the next week, cerebral function remained impaired, and she died.
Although the nature and pathogenesis of spontaneous HIT is still a matter of debate, this phenomenon exists, and in my opinion reflects the autoimmune-extreme of the anti-platelet factor 4 (PF4)/heparin immune response. This immune response ranges from nonplatelet-activating antibodies (enzyme-linked immunosorbent assay positive; even found in the general population and in up to 50% of non-HIT patients after cardiac surgery), to heparin-dependent platelet-activating IgG antibodies (typical for clinical HIT; but also found in up to 20% of non-HIT patients when screened after major surgery), to IgG antibodies that strongly activate platelets even in the absence of heparin. The latter pattern is typical for delayed onset and spontaneous HIT; but also, ∼30% of patients with typical HIT show this pattern during the first days after onset of HIT.
How could one understand and potentially explain this unusual spectrum? The immune response toward PF4/polyanion complexes is likely an ancient immune response pattern. PF4 and its receptor CXCR3B are preserved during evolution2 and the B cells producing these antibodies are marginal zone (MZ) B cells3 (at least in mice), which are also considered primitive B cells from an evolutionary viewpoint. MZ B cells can produce IgG antibodies without T-cell4 involvement but typically require additional danger signals to become activated. In addition, even normal individuals harbor B cells which, after in vitro stimulation, produce anti-PF4/heparin antibodies,5 and patients post liver transplant can produce anti-PF4/heparin IgG despite pharmacologic T-cell suppression.6 Both observations imply that, also in humans, MZ B cells are likely involved in producing anti-PF4/heparin IgG.
Several years ago, we showed that PF4 binds to polyanions on bacteria and that PF4-coated bacteria induce anti-PF4/heparin antibodies in mice, which facilitate opsonization of PF4-coated bacteria. We postulated that anti-PF4/polyanion antibodies are an ancient bacterial host defense mechanism, and that HIT results when this defense mechanism becomes misdirected7 toward PF4 on heparin-coated platelets (see figure, panels B-C).
In 2001, the phenomenon of “delayed onset HIT” was described by Warkentin and Kelton.8 Here, HIT begins (or worsens) after all heparin has been stopped; although the immune response is triggered by heparin, the B cells produce antibodies that bind to platelet-bound PF4 and induce platelet activation even in the absence of heparin, as shown by strong buffer reactivity in functional assays. Similarly-reacting antibodies are found in ∼30% of patients with acute HIT and, as shown by Warkentin et al,1 in the serum of patients with spontaneous HIT.
Sachais et al provided one potential explanation for these observations.9 The monoclonal antibody KKO, which recognizes PF4/heparin complexes, is able to cluster PF4 itself. In other words, this antibody creates its own (HIT) antigen(s). Antibodies in spontaneous HIT also recognize PF4 in the absence of heparin and, potentially, they are also able to cluster PF4, creating the antigen(s) to which HIT antibodies bind (although this needs to be shown experimentally).
It is unresolved whether this phenomenon just depends on the quantity of circulating anti-PF4/heparin antibodies or whether it is a qualitative characteristic of a subgroup of anti-PF4/heparin antibodies that are produced as a consequence of epitope spreading of B cells. A typical example of epitope spreading is posttransfusion purpura. Here, susceptible women, previously exposed to a platelet alloantigen (usually HPA-1a), develop high-titer anti-HPA-1a alloantibodies when they are boosted many years later by a blood transfusion containing HPA-1a platelets. These newly formed high-titer antibodies paradoxically do not follow “textbook immunology” but bind both HPA-1a–positive platelets as well as autologous HPA-1bb platelets, causing severe thrombocytopenia. Just as with spontaneous HIT and delayed onset HIT, these antibodies “broaden their specificity” but fortunately also disappear within a few weeks.
How is spontaneous HIT triggered? The frequently observed association between spontaneous HIT and recent infections or major surgery suggests the following concept: bacteria can bind PF4 and expose PF4 clusters2 ; PF4 also binds negatively charged nucleic acids, which are released during major surgery, forming PF4/nucleic acid complexes.10 Both mechanisms can thus generate “HIT antigens,” which in the setting of infection or major surgery induce “danger signals,” triggering B cells to produce the pathogenic anti-PF4/polyanion antibodies (see figure).
There are 3 major implications from spontaneous HIT.
First, should we test any patient with thrombocytopenia and thrombosis for anti-PF4/heparin antibodies? No! Such an approach would produce an avalanche of misdiagnoses and potentially dangerous overtreatment. However, in patients presenting with inexplicable thrombosis and thrombocytopenia, spontaneous HIT should be considered as a rare cause. As per the recommendations of Warkentin and colleagues,1 the diagnosis should only be made if there are high-titer anti-PF4/heparin IgG antibodies and a positive functional assay for HIT antibodies with the additional feature of a positive buffer control reaction. Second, in a patient who develops moderate thrombocytopenia during post surgical thrombosis prophylaxis with one of the new anticoagulants, spontaneous HIT should be excluded before the anticoagulant is stopped.
Third, systematic studies are needed focusing on the clinical course of HIT in patients showing a positive functional assay in the absence of heparin. Potentially, these patients could have more severe HIT.
Conflict-of-interest disclosure: The author declares no competing financial interests.