Key Points
Patients living in low SES neighborhoods had worse survival after DLBCL.
Disparity was most striking in younger (non-Medicare) patients and after the introduction of rituximab.
Abstract
Despite advances in treatment, including the introduction of rituximab, survival after diffuse large B-cell lymphoma (DLBCL) remains heterogeneous. However, no studies have considered the association between neighborhood socioeconomic status (SES) and race/ethnicity on DLBCL mortality before (1988-2000) and after (2001-2009) the introduction of rituximab. We studied all 33 032 DLBCL patients diagnosed between 1988-2009 in California for vital status through December 31, 2010. Patients diagnosed from 2001 to 2009 vs 1988 to 2000 had significantly decreased overall and DLBCL-specific mortality. However, those living in lower SES neighborhoods had 34% (95% confidence interval [CI], 27%-40%) and 24% (95% CI, 16%-32%) higher mortality rate from all causes and lymphoma, respectively, than patients in higher SES neighborhoods. The magnitude of mortality disparities by neighborhood SES was more marked in younger (<65 years) than in older patients (≥65 years), in married than nonmarried patients, and after 2000. We concluded that patients living in low SES neighborhoods had substantially worse survival after DLBCL, and this disparity was striking in younger (ie, not eligible for Medicare-aged) patients, married patients, and after the introduction of rituximab. These disparities suggest there are barriers, including inadequate insurance coverage with additional financial burden, to effective treatment among socioeconomically disadvantaged patients.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL), accounting for over 30% of all NHL.1,2 DLBCL is an aggressive histologic subtype of NHL, with rapidly enlarged lymph nodes, advanced-stage disease, and often “B” symptoms (ie, drenching night sweats, 10% weight loss, documented fever), with over 50% of untreated patients surviving <1 year.3 Intensive combination therapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) can achieve a complete remission in the majority of patients, a significant number of whom achieve long-term disease-free survival.4 Standard clinical prognostic models have identified age, stage, extranodal sites, performance status, and serum lactate dehydrogenase (LDH) level as being independently predictive of clinical outcome with intensive chemotherapy, and comprise the basis of the International Prognostic Index (IPI).5,6
Recently, a Surveillance, Epidemiology, and End Results (SEER) analysis reported that the median overall survival after DLBCL increased from >20 months for patients diagnosed before 2000 to 47 months after 2000.7 This remarkable improvement in survival coincided with the addition of rituximab to conventional chemotherapy regimens (R-CHOP therapy) in ∼2002.6,8 R-CHOP–based therapies effectively increased the overall 5-year relative survival from ∼45% to 60% to 70%,9,10 although worse survival was still noted for those with higher IPI scores.6
Despite these advances in treatment, African-American patients were reported to have risks of death 10% to 20% higher than non-Hispanic whites after DLBCL.7,11-13 SEER-Medicare analyses including patients treated predominantly in the CHOP era have suggested that delayed therapy or socioeconomic factors (specifically poverty, education, and family income) partially explain these results.11,14 However, no studies have considered how neighborhood socioeconomic status (SES) influences these racial/ethnic disparities in DLBCL despite SES being an important cancer prognostic factor15-18 and often underlying the associations between race/ethnicity and poor health outcomes.14,19 Therefore, this study aimed to understand whether socioeconomic disparities in DLBCL mortality exist and whether mortality improvements from advancements in DLBCL therapy exist across all patients after consideration for neighborhood SES. We examined the association between neighborhood SES and overall- and DLBCL-specific mortality before (1988-2000) and after (2001-2009) the introduction of rituximab among the ethnically and socioeconomically diverse population in the California Cancer Registry (CCR).
Patients and methods
Patients
We included all patients older than 15 years of age diagnosed with DLBCL (International Classification of Diseases-Oncology, 3rd edition [ICD-O-3] morphology codes 9680-968420-22 ) in California between January 1, 1988 and December 31, 2009. For each patient, we obtained CCR information on age, sex, race/ethnicity, marital status, census-block group of residence at diagnosis, summary stage, presence of “B” symptoms, nodal status (nodal defined as ICD-O-3 sites C024, C098-C099, C111, C142, C379, C422, C770-C779; extranodal defined as all other sites), treatment modalities within the first 12 months after diagnosis, vital status as of December 31, 2010, and underlying cause of death. Chemotherapy regimens were grouped as yes (received single agent, multiple agents, and chemotherapy not otherwise specified [NOS]), no (contraindicated, patient died, recommended but not given, refused, and none), and unknown (recommended but unknown if given, and unknown). Reporting hospitals were classified according to National Cancer Institute (NCI)-designated cancer center status.
We also obtained information on the primary source of payment at the time of initial diagnosis and/or treatment (health insurance), which was routinely abstracted by the CCR for patients diagnosed after 2001. Health insurance was grouped into public insurance (Medicaid and other government-assisted programs), private insurance (health maintenance organizations, preferred provider organizations, managed care NOS, and military care), no insurance and self-paid, and insurance status unknown.23
In addition, because individual patient-level SES information is not collected by the CCR, we determined neighborhood SES by patients’ residential census-block group using an index derived from principal components of 7 indicator variables of SES (education level; proportion unemployed and with a blue collar job; proportion <200% poverty line; and median household income, rent, and home value).24 DLBCL patients were assigned into an SES quintile based on the distribution of neighborhood SES across all census block groups in California. Based upon the population density of census blocks,25,26 we defined urban as those from metropolitan urban and metropolitan suburban blocks and those from city, town, and rural blocks as nonurban.
The introduction of monoclonal anti-CD20 antibody rituximab to treatment of B-cell NHL started in the late 1990s27,28 ; the addition of rituximab to conventional chemotherapy started in the late 1990s29,30 and became a consensus standard therapy after 2002.31 Given that survival data on rituximab use in combination with CHOP chemotherapy for treatment of DLBCL were first presented in 2000 and were rapidly adopted in clinical practice, we define this as the beginning of the modern treatment era. We excluded DLBCL patients with unknown race/ethnicity or age (n = 343) or, due to small numbers, patients who were American Indian or Alaska native (n = 118). We also excluded, in a hierarchical manner, patients with evidence of current HIV infection (CCR information on whether all Hodgkin and NHLs were associated with HIV/AIDS) or those who had died of AIDS (cause of death information coded by the National Center for Health Statistics and the California Department of Public Health based on information from the death certificate) (n = 3218) for whom treatment and survival patterns were known to be different, and all patients diagnosed at autopsy or by death certificate (n = 137) with zero survival time. The final study population included 33 032 patients. This project was approved by the Institutional Review Board of the Cancer Prevention Institute of California.
Statistical analysis
We used Cox proportional hazards regression to estimate survival hazard ratios (HRs) and associated 95% confidence intervals (CIs) to evaluate the impact of factors on overall and DLBCL-specific mortality. For deceased patients, survival time was measured in days from the date of diagnosis to the date of death from any cause for analyses of all-cause mortality or to the date of death from DLBCL for analyses of DLBCL-specific mortality. Patients who died of other causes were censored at the time of death for analyses of DLBCL-specific death. Patients alive at the study end date (December 31, 2010) were censored at this time or at the date of last follow-up (ie, last known contact). Ninety-three percent of censored patients had a follow-up date within 1 year of the study end date; neighborhood SES did not differ between patients with and without recent follow-up information.
We tested proportional hazards assumption by statistically testing the correlation between weighted Schoenfeld residuals and logarithmically transformed survival time. No violations of the assumption were observed. Multivariate Cox regression models included variables significant at P < .05 in unadjusted models (age at diagnosis, race/ethnicity, marital status, diagnostic period, “B” symptoms, nodal status, chemotherapy, radiation therapy, NCI-designated hospital, and neighborhood SES), or with a priori hypotheses for inclusion (eg, sex and urbanicity). Given that proportional hazards varied by stage at diagnosis (localized, regional, advanced, and unknown), it was included as a stratifying variable in the analyses and is not included in Table 2. Multivariate models also included source of health insurance coverage to examine the impact of health insurance on mortality in subanalysis for DLBCL patients diagnosed after 2001. Effect modification between neighborhood SES quintile and age at diagnosis, stage, period of diagnosis, and other factors was assessed by including interaction terms in the multivariable models and was considered present if the interaction term was significant at P < .05. A significant interaction was found between neighborhood SES and age group (<65 and ≥65 years), period of diagnosis, and marital status at diagnosis (nonmarried [never married, separated, divorced, and widowed] and married). Further stratification analyses were conducted to determine whether mortality differed for patient subgroups defined by age group, marital status, stage, and period of diagnosis. The Kaplan-Meier method and log-rank test were used to compare unadjusted overall- and DLBCL-specific survival in patients defined by stage at diagnosis and neighborhood SES, which were collapsed into 2 groups (quintiles 1-2 [lower SES], and quintiles 3-5 [higher SES]) due to similarities across quintiles in survival patterns.
All statistical tests were carried out using SAS software version 9.3 (SAS Institute). All P values reported were 2-sided, and those that were <.05 were considered to be statistically significant.
Results
In this California cohort of 33 032 DLBCL patients, the mean age (±standard deviation) of patients at diagnosis was 63.3 (±16.9) years [63.0 (±17.0) years and 63.6 (±16.9) years for patients diagnosed before and after 2000, respectively]. Table 1 shows that most patients in our study were of non-Hispanic white race/ethnicity, but the proportion of nonwhite patients increased in the 2001 to 2009 diagnostic period.
Demographic and clinical characteristics of patients with DLBCL by period of diagnosis in California, 1988-2009
| Characteristics . | Period of diagnosis, no. (%) . | P . | |
|---|---|---|---|
| 1988-2000, N = 16 899 . | 2001-2009, N = 16 133 . | ||
| Age at diagnosis, y | |||
| 75+ | 4 791 (28.4) | 4 984 (30.9) | <.01 |
| 65-74 | 4 354 (25.8) | 3 513 (21.8) | |
| 55-64 | 2 923 (17.3) | 3 100 (19.2) | |
| 45-54 | 2 087 (12.3) | 2 236 (13.9) | |
| 0-44 | 2 744 (16.2) | 2 300 (14.3) | |
| Race/ethnicity | |||
| Non-Hispanic white | 12 133 (71.8) | 9 950 (61.7) | <.01 |
| African Americans | 670 (4.0) | 718 (4.5) | |
| Hispanic | 2 556 (15.1) | 3 492 (21.6) | |
| Asian/Pacific Islander | 1 540 (9.1) | 1 973 (12.2) | |
| Sex | .54 | ||
| Male | 8 975 (53.1) | 8 623 (53.4) | |
| Female | 7 924 (46.9) | 7 510 (46.6) | |
| Marital status at diagnosis | <.01 | ||
| Married | 9 867 (58.4) | 8 998 (55.8) | |
| Never married | 2 281 (13.5) | 2 787 (17.3) | |
| Previously married | 4 279 (25.3) | 3 843 (23.8) | |
| Unknown | 472 (2.8) | 505 (3.1) | |
| Stage at diagnosis | <.01 | ||
| Localized | 4 760 (28.2) | 4 134 (25.6) | |
| Regional | 3 651 (21.6) | 3 347 (20.7) | |
| Advanced | 7 161 (42.4) | 7 552 (46.8) | |
| Unknown | 1 327 (7.9) | 1 100 (6.8) | |
| B symptoms | <.01 | ||
| Absent | 5 517 (32.6) | 7 916 (49.1) | |
| Present | 3 433 (20.3) | 4 283 (26.5) | |
| Unknown | 7 949 (47.0) | 3 934 (24.4) | |
| Nodal status | .13 | ||
| Nodal | 11 343 (67.1) | 10 703 (66.3) | |
| Extranodal | 5 556 (32.9) | 5 430 (33.7) | |
| Chemotherapy | .03 | ||
| No | 3 829 (22.7) | 3 805 (23.6) | |
| Yes | 12 717 (75.3) | 12 036 (74.6) | |
| Unknown | 353 (2.1) | 292 (1.8) | |
| Radiation | <.01 | ||
| No | 12 112 (71.7) | 12 671 (78.5) | |
| Yes | 4 784 (28.3) | 3 460 (21.4) | |
| Unknown | <5 | <5 | |
| Treated at an NCI-designated hospital | <.01 | ||
| No | 15 671 (92.7) | 14 637 (90.7) | |
| Yes | 1 227 (7.3) | 1 495 (9.3) | |
| Unknown | <5 | <5 | |
| Urbanicity | |||
| Urban | 11 298 (66.9) | 10 409 (64.5) | |
| Nonurban | 4 930 (29.2) | 5 243 (32.5) | |
| Unknown | 671 (4.0) | 481 (3.0) | <.01 |
| Neighborhood SES quintile | .62 | ||
| 1. Lowest 20% | 2 385 (14.1) | 2 374 (14.7) | |
| 2 | 3 073 (18.2) | 2 940 (18.2) | |
| 3 | 3 530 (20.9) | 3 349 (20.8) | |
| 4 | 3 804 (22.5) | 3 581 (22.2) | |
| 5. Highest 20% | 4 107 (24.3) | 3 889 (24.1) | |
| Characteristics . | Period of diagnosis, no. (%) . | P . | |
|---|---|---|---|
| 1988-2000, N = 16 899 . | 2001-2009, N = 16 133 . | ||
| Age at diagnosis, y | |||
| 75+ | 4 791 (28.4) | 4 984 (30.9) | <.01 |
| 65-74 | 4 354 (25.8) | 3 513 (21.8) | |
| 55-64 | 2 923 (17.3) | 3 100 (19.2) | |
| 45-54 | 2 087 (12.3) | 2 236 (13.9) | |
| 0-44 | 2 744 (16.2) | 2 300 (14.3) | |
| Race/ethnicity | |||
| Non-Hispanic white | 12 133 (71.8) | 9 950 (61.7) | <.01 |
| African Americans | 670 (4.0) | 718 (4.5) | |
| Hispanic | 2 556 (15.1) | 3 492 (21.6) | |
| Asian/Pacific Islander | 1 540 (9.1) | 1 973 (12.2) | |
| Sex | .54 | ||
| Male | 8 975 (53.1) | 8 623 (53.4) | |
| Female | 7 924 (46.9) | 7 510 (46.6) | |
| Marital status at diagnosis | <.01 | ||
| Married | 9 867 (58.4) | 8 998 (55.8) | |
| Never married | 2 281 (13.5) | 2 787 (17.3) | |
| Previously married | 4 279 (25.3) | 3 843 (23.8) | |
| Unknown | 472 (2.8) | 505 (3.1) | |
| Stage at diagnosis | <.01 | ||
| Localized | 4 760 (28.2) | 4 134 (25.6) | |
| Regional | 3 651 (21.6) | 3 347 (20.7) | |
| Advanced | 7 161 (42.4) | 7 552 (46.8) | |
| Unknown | 1 327 (7.9) | 1 100 (6.8) | |
| B symptoms | <.01 | ||
| Absent | 5 517 (32.6) | 7 916 (49.1) | |
| Present | 3 433 (20.3) | 4 283 (26.5) | |
| Unknown | 7 949 (47.0) | 3 934 (24.4) | |
| Nodal status | .13 | ||
| Nodal | 11 343 (67.1) | 10 703 (66.3) | |
| Extranodal | 5 556 (32.9) | 5 430 (33.7) | |
| Chemotherapy | .03 | ||
| No | 3 829 (22.7) | 3 805 (23.6) | |
| Yes | 12 717 (75.3) | 12 036 (74.6) | |
| Unknown | 353 (2.1) | 292 (1.8) | |
| Radiation | <.01 | ||
| No | 12 112 (71.7) | 12 671 (78.5) | |
| Yes | 4 784 (28.3) | 3 460 (21.4) | |
| Unknown | <5 | <5 | |
| Treated at an NCI-designated hospital | <.01 | ||
| No | 15 671 (92.7) | 14 637 (90.7) | |
| Yes | 1 227 (7.3) | 1 495 (9.3) | |
| Unknown | <5 | <5 | |
| Urbanicity | |||
| Urban | 11 298 (66.9) | 10 409 (64.5) | |
| Nonurban | 4 930 (29.2) | 5 243 (32.5) | |
| Unknown | 671 (4.0) | 481 (3.0) | <.01 |
| Neighborhood SES quintile | .62 | ||
| 1. Lowest 20% | 2 385 (14.1) | 2 374 (14.7) | |
| 2 | 3 073 (18.2) | 2 940 (18.2) | |
| 3 | 3 530 (20.9) | 3 349 (20.8) | |
| 4 | 3 804 (22.5) | 3 581 (22.2) | |
| 5. Highest 20% | 4 107 (24.3) | 3 889 (24.1) | |
With a mean follow-up of 4.5 (±5.1) years [5.8 (±6.4) years and 3.1 (±2.8) years, respectively, for patients diagnosed before and after 2000], the median overall survival was 7.10 years (95% CI, 6.80-7.42) for patients with regional/localized disease, and 1.48 years (95% CI, 1.40-1.57) for those with advanced disease. Table 2 shows that DLBCL patients diagnosed in 2001 to 2009 had 29% to 32% lower mortality compared with patients diagnosed in 1988 to 2000. Female patients experienced lower mortality than male patients. Significantly increased mortality risk was observed in patients who were not married, with advanced stage, presence of “B” symptoms, and living in the lower SES neighborhoods (all P values < .05; P for trend < .01). Compared with patients living in the top 20% of neighborhoods ranked by SES, patients in the lowest 20% experienced 34% (95% CI, 27%-40%) increased risk of all-cause death and 24% (95% CI, 16%-32%) increased risk of DLBCL-specific death.
| Characteristics . | All-cause mortality . | DLBCL-specific mortality . | ||
|---|---|---|---|---|
| No. of deaths . | HR (95% CI) . | No. of deaths . | HR (95% CI) . | |
| Age at diagnosis | 1.04 (1.04-1.04) | 1.03 (1.03-1.03) | ||
| Race/ethnicity | ||||
| Non-Hispanic white | 14 148 | 1.00 (Reference) | 9 008 | 1.00 (Reference) |
| African American | 787 | 1.00 (0.93-1.08) | 489 | 0.94 (0.86-1.03) |
| Hispanic | 3 405 | 1.04 (1.00-1.08) | 2 193 | 1.02 (0.97-1.07) |
| Asian/Pacific Islander | 1 979 | 1.03 (0.98-1.08) | 1 338 | 1.08 (1.02-1.15) |
| Sex | ||||
| Male | 10 819 | 1.00 (Reference) | 6 792 | 1.00 (Reference) |
| Female | 9 500 | 0.82 (0.79-0.84) | 6 236 | 0.90 (0.87-0.94) |
| Marital status at diagnosis | ||||
| Married | 11 222 | 1.00 (Reference) | 7 278 | 1.00 (Reference) |
| Never married | 2 482 | 1.22 (1.16-1.27) | 1 611 | 1.13 (1.07-1.20) |
| Previously married | 6 061 | 1.19 (1.15-1.23) | 3 804 | 1.13 (1.09-1.18) |
| Unknown | 554 | 0.97 (0.89-1.06) | 335 | 0.91 (0.81-1.01) |
| Period of diagnosis | ||||
| 1988-2000 | 12 832 | 1.00 (Reference) | 7 838 | 1.00 (Reference) |
| 2001-2009 | 7 487 | 0.71 (0.68-0.73) | 5 190 | 0.69 (0.67-0.72) |
| B symptoms | ||||
| Absent | 7 090 | 1.00 (Reference) | 4 427 | 1.00 (Reference) |
| Present | 4 832 | 1.33 (1.28-1.38) | 3 511 | 1.45 (1.38-1.52) |
| Unknown | 8 397 | 1.12 (1.09-1.16) | 5 090 | 1.14 (1.09-1.19) |
| Nodal status | ||||
| Nodal | 13 638 | 1.00 (Reference) | 9 110 | 1.00 (Reference) |
| Extranodal | 6 681 | 1.01 (0.98-1.04) | 3 918 | 0.97 (0.93-1.01) |
| Chemotherapy | ||||
| No | 5 815 | 1.00 (Reference) | 3 461 | 1.00 (Reference) |
| Yes | 14 117 | 0.52 (0.50-0.54) | 9 339 | 0.53 (0.51-0.55) |
| Unknown | 387 | 0.52 (0.47-0.58) | 228 | 0.52 (0.45-0.59) |
| Radiation | ||||
| No | 15 742 | 1.00 (Reference) | 10 230 | 1.00 (Reference) |
| Yes | 4 573 | 0.84 (0.81-0.87) | 2 795 | 0.85 (0.81-0.89) |
| Treated at an NCI-designated hospital | ||||
| No | 18 936 | 1.00 (Reference) | 12 148 | 1.00 (Reference) |
| Yes | 1 382 | 0.96 (0.90-1.01) | 880 | 0.90 (0.84-0.96) |
| Urbanicity | ||||
| Urban | 13 351 | 1.00 (Reference) | 8 629 | 1.00 (Reference) |
| Nonurban | 6 256 | 0.96 (0.93-0.99) | 3 977 | 0.95 (0.91-0.99) |
| Unknown | 712 | 0.93 (0.86-1.00) | 422 | 0.89 (0.81-0.99) |
| Neighborhood SES quintile | ||||
| 1. Lowest 20% | 3 079 | 1.34 (1.27-1.40) | 1 905 | 1.24 (1.16-1.32) |
| 2 | 3 885 | 1.26 (1.20-1.32) | 2 525 | 1.23 (1.16-1.30) |
| 3 | 4 295 | 1.17 (1.12-1.22) | 2 731 | 1.12 (1.06-1.18) |
| 4 | 4 528 | 1.09 (1.05-1.14) | 2 915 | 1.07 (1.02-1.13) |
| 5. Highest 20% | 4 532 | 1.00 (Reference) | 2 952 | 1.00 (Reference) |
| Ptrend < 0.01 | Ptrend < 0.01 | |||
| Limited to patients who were diagnosed after 2001 and were under the age of 65 y | ||||
| Insurance status‡ | ||||
| Private/military insurance | 1 192 | 1.00 (Reference) | 895 | 1.00 (Reference) |
| Public insurance/no insurance | 846 | 1.54 (1.40-1.69) | 597 | 1.46 (1.30-1.63) |
| Unknown | 240 | 1.00 (0.87-1.15) | 184 | 1.07 (0.91-1.25) |
| Characteristics . | All-cause mortality . | DLBCL-specific mortality . | ||
|---|---|---|---|---|
| No. of deaths . | HR (95% CI) . | No. of deaths . | HR (95% CI) . | |
| Age at diagnosis | 1.04 (1.04-1.04) | 1.03 (1.03-1.03) | ||
| Race/ethnicity | ||||
| Non-Hispanic white | 14 148 | 1.00 (Reference) | 9 008 | 1.00 (Reference) |
| African American | 787 | 1.00 (0.93-1.08) | 489 | 0.94 (0.86-1.03) |
| Hispanic | 3 405 | 1.04 (1.00-1.08) | 2 193 | 1.02 (0.97-1.07) |
| Asian/Pacific Islander | 1 979 | 1.03 (0.98-1.08) | 1 338 | 1.08 (1.02-1.15) |
| Sex | ||||
| Male | 10 819 | 1.00 (Reference) | 6 792 | 1.00 (Reference) |
| Female | 9 500 | 0.82 (0.79-0.84) | 6 236 | 0.90 (0.87-0.94) |
| Marital status at diagnosis | ||||
| Married | 11 222 | 1.00 (Reference) | 7 278 | 1.00 (Reference) |
| Never married | 2 482 | 1.22 (1.16-1.27) | 1 611 | 1.13 (1.07-1.20) |
| Previously married | 6 061 | 1.19 (1.15-1.23) | 3 804 | 1.13 (1.09-1.18) |
| Unknown | 554 | 0.97 (0.89-1.06) | 335 | 0.91 (0.81-1.01) |
| Period of diagnosis | ||||
| 1988-2000 | 12 832 | 1.00 (Reference) | 7 838 | 1.00 (Reference) |
| 2001-2009 | 7 487 | 0.71 (0.68-0.73) | 5 190 | 0.69 (0.67-0.72) |
| B symptoms | ||||
| Absent | 7 090 | 1.00 (Reference) | 4 427 | 1.00 (Reference) |
| Present | 4 832 | 1.33 (1.28-1.38) | 3 511 | 1.45 (1.38-1.52) |
| Unknown | 8 397 | 1.12 (1.09-1.16) | 5 090 | 1.14 (1.09-1.19) |
| Nodal status | ||||
| Nodal | 13 638 | 1.00 (Reference) | 9 110 | 1.00 (Reference) |
| Extranodal | 6 681 | 1.01 (0.98-1.04) | 3 918 | 0.97 (0.93-1.01) |
| Chemotherapy | ||||
| No | 5 815 | 1.00 (Reference) | 3 461 | 1.00 (Reference) |
| Yes | 14 117 | 0.52 (0.50-0.54) | 9 339 | 0.53 (0.51-0.55) |
| Unknown | 387 | 0.52 (0.47-0.58) | 228 | 0.52 (0.45-0.59) |
| Radiation | ||||
| No | 15 742 | 1.00 (Reference) | 10 230 | 1.00 (Reference) |
| Yes | 4 573 | 0.84 (0.81-0.87) | 2 795 | 0.85 (0.81-0.89) |
| Treated at an NCI-designated hospital | ||||
| No | 18 936 | 1.00 (Reference) | 12 148 | 1.00 (Reference) |
| Yes | 1 382 | 0.96 (0.90-1.01) | 880 | 0.90 (0.84-0.96) |
| Urbanicity | ||||
| Urban | 13 351 | 1.00 (Reference) | 8 629 | 1.00 (Reference) |
| Nonurban | 6 256 | 0.96 (0.93-0.99) | 3 977 | 0.95 (0.91-0.99) |
| Unknown | 712 | 0.93 (0.86-1.00) | 422 | 0.89 (0.81-0.99) |
| Neighborhood SES quintile | ||||
| 1. Lowest 20% | 3 079 | 1.34 (1.27-1.40) | 1 905 | 1.24 (1.16-1.32) |
| 2 | 3 885 | 1.26 (1.20-1.32) | 2 525 | 1.23 (1.16-1.30) |
| 3 | 4 295 | 1.17 (1.12-1.22) | 2 731 | 1.12 (1.06-1.18) |
| 4 | 4 528 | 1.09 (1.05-1.14) | 2 915 | 1.07 (1.02-1.13) |
| 5. Highest 20% | 4 532 | 1.00 (Reference) | 2 952 | 1.00 (Reference) |
| Ptrend < 0.01 | Ptrend < 0.01 | |||
| Limited to patients who were diagnosed after 2001 and were under the age of 65 y | ||||
| Insurance status‡ | ||||
| Private/military insurance | 1 192 | 1.00 (Reference) | 895 | 1.00 (Reference) |
| Public insurance/no insurance | 846 | 1.54 (1.40-1.69) | 597 | 1.46 (1.30-1.63) |
| Unknown | 240 | 1.00 (0.87-1.15) | 184 | 1.07 (0.91-1.25) |
Cox models were adjusted for all of the characteristics shown in the table except for insurance status.
Stage at diagnosis (localized, regional, advanced, and unknown) was included as a stratifying variable.
Public insurance included Medicaid and other government-assisted programs; and private insurance included health maintenance organizations, preferred provider organizations, managed care NOS, and military care.
Relative to non-Hispanic whites (and after adjustment for age at diagnosis, sex, marital status, diagnostic period, stage, presence of “B” symptoms, nodal status, NCI-designated hospital, urbanicity, and first course of treatment), Hispanics (HR = 1.13; 95% CI, 1.08-1.17), Asian/Pacific Islanders (HR = 1.07; 95% CI, 1.02-1.12), and African Americans (HR = 1.09; 95% CI, 1.01-1.17) experienced higher all-cause mortality. Hispanics (HR = 1.08; 95% CI, 1.03-1.13) and Asians/Pacific Islanders (HR = 1.11; 95% CI, 1.05-1.18), but not African Americans (HR = 1.00; 95% CI, 0.92-1.10), also experienced higher DLBCL-specific mortality relative to non-Hispanic whites. Notably, after further adjustment for neighborhood SES, mortality was similar across all racial/ethnicity groups, except for an 8% higher DLBCL-specific mortality among Asian/Pacific Islanders (Table 2).
We also examined the association between the source of health insurance and risk of death in the 7636 patients under the age of 65 years diagnosed after 2001 (Table 2). Patients who were covered by government-assisted programs (n = 1697) or self-paid/without insurance (n = 379) experienced a 1.5-fold increased risk of death compared with patients who had managed care or military care (n = 4653) after adjusting for all factors. Adjusting for insurance status did not change the association between neighborhood SES and mortality risk.
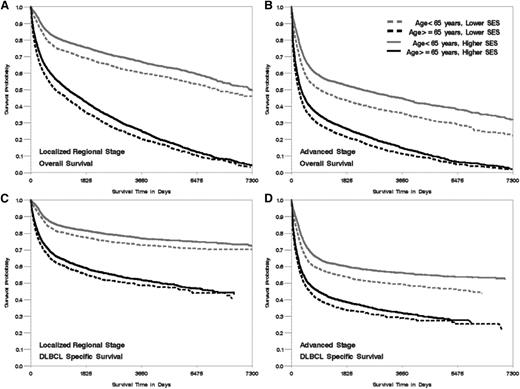
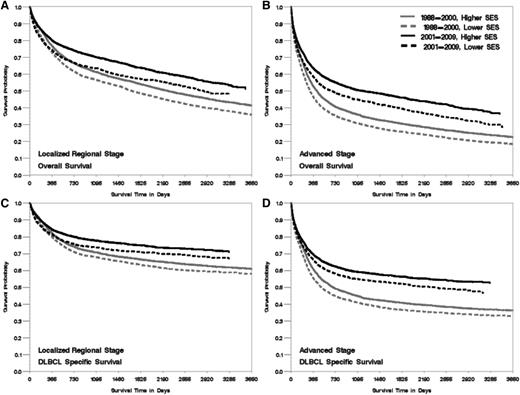
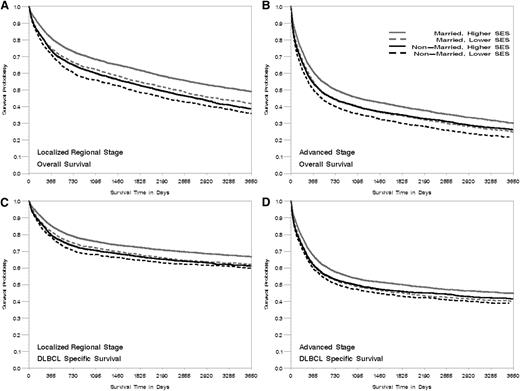
Patients living in lower SES neighborhoods had worse survival than those in higher SES neighborhoods across groups stratified by age, marital status, year of diagnosis, and stage at diagnosis (all Plog-rank < .005, Figures 1-3), with the effect of neighborhood SES on survival most striking in patients <65 years, those who are married, and those diagnosed after 2001 (Table 3). Patients living in the lowest SES neighborhoods who were diagnosed in the period from 2001 to 2009 had a 37% and 25% greater risk of death from all-cause and DLBCL, respectively, compared with patients in the highest SES neighborhood, whereas elevated risk was 31% and 23% for patients diagnosed from 1988 to 2000 (P for interaction = .01 for overall mortality). Among nonmarried patients, those living in lower SES neighborhoods had a 28% and 17% elevated risk of all-cause and DLBCL-specific mortality, respectively, compared with those residing in high SES neighborhoods. A stronger effect was noted among married patients, with a 34% and 26% elevated mortality risk observed for those living in lower SES neighborhoods compared with high SES neighborhoods (P for interaction for overall- and DLBCL-specific mortality <.01). The absolute magnitude of survival difference between the lowest and highest neighborhood SES was most marked in patients <65 years of age (51% and 49% increased risk for death from all causes and from DLBCL) (Table 3) (P for interaction for overall- and DLBCL-specific mortality <.01).
Kaplan-Meier curve of overall- and DLBCL-specific survival by age at diagnosis, neighborhood SES group, and stage at diagnosis, California 1988 to 2009. The vertical axis represents survival probability; the horizontal axis represents survival time in days. Lower SES refers to quintiles 1 and 2, and higher SES refers to quintiles 3 to 5. (A) Localized and regional stage, overall survival. (B) Advanced stage, overall survival. (C) Localized and regional stage, DLBCL-specific survival. (D) Advanced stage, DLBCL-specific survival. Plog-rank < .005.
Kaplan-Meier curve of overall- and DLBCL-specific survival by age at diagnosis, neighborhood SES group, and stage at diagnosis, California 1988 to 2009. The vertical axis represents survival probability; the horizontal axis represents survival time in days. Lower SES refers to quintiles 1 and 2, and higher SES refers to quintiles 3 to 5. (A) Localized and regional stage, overall survival. (B) Advanced stage, overall survival. (C) Localized and regional stage, DLBCL-specific survival. (D) Advanced stage, DLBCL-specific survival. Plog-rank < .005.
Kaplan-Meier curve of overall- and DLBCL-specific survival by period of diagnosis, neighborhood SES group, and stage at diagnosis, California. The vertical axis represents survival probability; the horizontal axis represents survival time in days. Lower SES refers to quintiles 1 and 2, and higher SES refers to quintiles 3 to 5. (A) Localized and regional stage, overall survival. (B) Advanced stage, overall survival. (C) Localized and regional stage, DLBCL-specific survival. (D) Advanced stage, DLBCL-specific survival. Plog-rank < .005.
Kaplan-Meier curve of overall- and DLBCL-specific survival by period of diagnosis, neighborhood SES group, and stage at diagnosis, California. The vertical axis represents survival probability; the horizontal axis represents survival time in days. Lower SES refers to quintiles 1 and 2, and higher SES refers to quintiles 3 to 5. (A) Localized and regional stage, overall survival. (B) Advanced stage, overall survival. (C) Localized and regional stage, DLBCL-specific survival. (D) Advanced stage, DLBCL-specific survival. Plog-rank < .005.
Kaplan-Meier curve of overall- and DLBCL-specific survival by marital status at diagnosis, neighborhood SES group, and stage at diagnosis, California 1988 to 2009. The vertical axis represents survival probability; the horizontal axis represents survival time in days. Lower SES refers to quintiles 1 and 2, and higher SES refers to quintiles 3 to 5. (A) Localized and regional stage, overall survival. (B) Advanced stage, overall survival. (C) Localized and regional stage, DLBCL-specific survival. (D) Advanced stage, DLBCL-specific survival. Plog-rank < .005.
Kaplan-Meier curve of overall- and DLBCL-specific survival by marital status at diagnosis, neighborhood SES group, and stage at diagnosis, California 1988 to 2009. The vertical axis represents survival probability; the horizontal axis represents survival time in days. Lower SES refers to quintiles 1 and 2, and higher SES refers to quintiles 3 to 5. (A) Localized and regional stage, overall survival. (B) Advanced stage, overall survival. (C) Localized and regional stage, DLBCL-specific survival. (D) Advanced stage, DLBCL-specific survival. Plog-rank < .005.
Multivariate adjusted* HR and 95% CI estimates for death from all causes and from lymphoma for SES quintiles stratified by period of diagnosis, stage at diagnosis, age group at diagnosis, and marital status, California 1988-2009
| . | All-cause mortality . | DLBCL-specific mortality . | ||||
|---|---|---|---|---|---|---|
| No. of deaths . | HR (95% CI) . | Pinteraction . | No. of deaths . | HR (95% CI) . | Pinteraction . | |
| Period of diagnosis | ||||||
| 1988-2000 | .01 | .29 | ||||
| 1. SES lowest 20% | 1875 | 1.31 (1.23-1.40) | 1115 | 1.23 (1.14-1.34) | ||
| 2 | 2420 | 1.24 (1.17-1.31) | 1501 | 1.22 (1.13-1.31) | ||
| 3 | 2746 | 1.19 (1.13-1.26) | 1665 | 1.14 (1.07-1.22) | ||
| 4 | 2887 | 1.10 (1.04-1.16) | 1773 | 1.08 (1.01-1.16) | ||
| 5. SES highest 20% | 2904 | 1.00 (Reference) | 1784 | 1.00 (Reference) | ||
| 2001-2009 | ||||||
| 1. SES lowest 20% | 1204 | 1.37 (1.27-1.49) | 790 | 1.25 (1.14-1.38) | ||
| 2 | 1465 | 1.29 (1.20-1.39) | 1024 | 1.26 (1.15-1.37) | ||
| 3 | 1549 | 1.14 (1.06-1.23) | 1066 | 1.10 (1.01-1.20) | ||
| 4 | 1641 | 1.08 (1.01-1.16) | 1142 | 1.06 (0.97-1.15) | ||
| 5. SES highest 20% | 1628 | 1.00 (Reference) | 1168 | 1.00 (Reference) | ||
| Stage at diagnosis | .11 | .14 | ||||
| Localized/regional stage | ||||||
| 1. SES lowest 20% | 1219 | 1.29 (1.19-1.39) | 663 | 1.19 (1.07-1.31) | ||
| 2 | 1609 | 1.22 (1.14-1.31) | 939 | 1.21 (1.10-1.32) | ||
| 3 | 1816 | 1.15 (1.08-1.22) | 1017 | 1.09 (1.00-1.19) | ||
| 4 | 1941 | 1.10 (1.03-1.17) | 1139 | 1.10 (1.01-1.19) | ||
| 5. SES highest 20% | 2012 | 1.00 (Reference) | 1169 | 1.00 (Reference) | ||
| Advanced stage | ||||||
| 1. SES lowest 20% | 1593 | 1.32 (1.23-1.41) | 1093 | 1.23 (1.13-1.33) | ||
| 2 | 1945 | 1.24 (1.17-1.32) | 1385 | 1.20 (1.11-1.29) | ||
| 3 | 2123 | 1.14 (1.07-1.21) | 1514 | 1.11 (1.03-1.19) | ||
| 4 | 2213 | 1.06 (1.00-1.12) | 1569 | 1.03 (0.96-1.10) | ||
| 5. SES highest 20% | 2246 | 1.00 (Reference) | 1626 | 1.00 (Reference) | ||
| Age at diagnosis, y | <.01 | <.01 | ||||
| 15-64 | ||||||
| 1. SES lowest 20% | 1219 | 1.51 (1.39-1.64) | 811 | 1.49 (1.34-1.65) | ||
| 2 | 1321 | 1.38 (1.27-1.49) | 925 | 1.41 (1.28-1.55) | ||
| 3 | 1379 | 1.22 (1.13-1.32) | 955 | 1.24 (1.13-1.35) | ||
| 4 | 1437 | 1.16 (1.08-1.25) | 982 | 1.16 (1.06-1.27) | ||
| 5. SES highest 20% | 1416 | 1.00 (Reference) | 958 | 1.00 (Reference) | ||
| 65+ | ||||||
| 1. SES lowest 20% | 1860 | 1.26 (1.19-1.34) | 1094 | 1.13 (1.04-1.22) | ||
| 2 | 2564 | 1.21 (1.15-1.28) | 1600 | 1.16 (1.08-1.24) | ||
| 3 | 2916 | 1.16 (1.10-1.22) | 1776 | 1.08 (1.01-1.15) | ||
| 4 | 3091 | 1.06 (1.01-1.12) | 1933 | 1.03 (0.97-1.10) | ||
| 5. SES highest 20% | 3116 | 1.00 (Reference) | 1994 | 1.00 (Reference) | ||
| Marital status at diagnosis | <.01 | <.01 | ||||
| Nonmarried (never married and previously married) | ||||||
| 1. SES lowest 20% | 1557 | 1.28 (1.19-1.38) | 942 | 1.17 (1.06-1.28) | ||
| 2 | 1683 | 1.17 (1.09-1.25) | 1082 | 1.13 (1.04-1.24) | ||
| 3 | 1807 | 1.06 (1.00-1.14) | 1139 | 1.03 (0.94-1.12) | ||
| 4 | 1842 | 1.01 (0.95-1.08) | 1180 | 1.00 (0.92-1.09) | ||
| 5. SES highest 20% | 1654 | 1.00 (Reference) | 1072 | 1.00 (Reference) | ||
| Married | ||||||
| 1. SES lowest 20% | 1436 | 1.34 (1.25-1.44) | 910 | 1.26 (1.16-1.38) | ||
| 2 | 2108 | 1.30 (1.23-1.38) | 1393 | 1.29 (1.20-1.39) | ||
| 3 | 2367 | 1.23 (1.16-1.30) | 1517 | 1.18 (1.10-1.26) | ||
| 4 | 2535 | 1.13 (1.07-1.20) | 1641 | 1.10 (1.03-1.18) | ||
| 5. SES highest 20% | 2776 | 1.00 (Reference) | 1817 | 1.00 (Reference) | ||
| . | All-cause mortality . | DLBCL-specific mortality . | ||||
|---|---|---|---|---|---|---|
| No. of deaths . | HR (95% CI) . | Pinteraction . | No. of deaths . | HR (95% CI) . | Pinteraction . | |
| Period of diagnosis | ||||||
| 1988-2000 | .01 | .29 | ||||
| 1. SES lowest 20% | 1875 | 1.31 (1.23-1.40) | 1115 | 1.23 (1.14-1.34) | ||
| 2 | 2420 | 1.24 (1.17-1.31) | 1501 | 1.22 (1.13-1.31) | ||
| 3 | 2746 | 1.19 (1.13-1.26) | 1665 | 1.14 (1.07-1.22) | ||
| 4 | 2887 | 1.10 (1.04-1.16) | 1773 | 1.08 (1.01-1.16) | ||
| 5. SES highest 20% | 2904 | 1.00 (Reference) | 1784 | 1.00 (Reference) | ||
| 2001-2009 | ||||||
| 1. SES lowest 20% | 1204 | 1.37 (1.27-1.49) | 790 | 1.25 (1.14-1.38) | ||
| 2 | 1465 | 1.29 (1.20-1.39) | 1024 | 1.26 (1.15-1.37) | ||
| 3 | 1549 | 1.14 (1.06-1.23) | 1066 | 1.10 (1.01-1.20) | ||
| 4 | 1641 | 1.08 (1.01-1.16) | 1142 | 1.06 (0.97-1.15) | ||
| 5. SES highest 20% | 1628 | 1.00 (Reference) | 1168 | 1.00 (Reference) | ||
| Stage at diagnosis | .11 | .14 | ||||
| Localized/regional stage | ||||||
| 1. SES lowest 20% | 1219 | 1.29 (1.19-1.39) | 663 | 1.19 (1.07-1.31) | ||
| 2 | 1609 | 1.22 (1.14-1.31) | 939 | 1.21 (1.10-1.32) | ||
| 3 | 1816 | 1.15 (1.08-1.22) | 1017 | 1.09 (1.00-1.19) | ||
| 4 | 1941 | 1.10 (1.03-1.17) | 1139 | 1.10 (1.01-1.19) | ||
| 5. SES highest 20% | 2012 | 1.00 (Reference) | 1169 | 1.00 (Reference) | ||
| Advanced stage | ||||||
| 1. SES lowest 20% | 1593 | 1.32 (1.23-1.41) | 1093 | 1.23 (1.13-1.33) | ||
| 2 | 1945 | 1.24 (1.17-1.32) | 1385 | 1.20 (1.11-1.29) | ||
| 3 | 2123 | 1.14 (1.07-1.21) | 1514 | 1.11 (1.03-1.19) | ||
| 4 | 2213 | 1.06 (1.00-1.12) | 1569 | 1.03 (0.96-1.10) | ||
| 5. SES highest 20% | 2246 | 1.00 (Reference) | 1626 | 1.00 (Reference) | ||
| Age at diagnosis, y | <.01 | <.01 | ||||
| 15-64 | ||||||
| 1. SES lowest 20% | 1219 | 1.51 (1.39-1.64) | 811 | 1.49 (1.34-1.65) | ||
| 2 | 1321 | 1.38 (1.27-1.49) | 925 | 1.41 (1.28-1.55) | ||
| 3 | 1379 | 1.22 (1.13-1.32) | 955 | 1.24 (1.13-1.35) | ||
| 4 | 1437 | 1.16 (1.08-1.25) | 982 | 1.16 (1.06-1.27) | ||
| 5. SES highest 20% | 1416 | 1.00 (Reference) | 958 | 1.00 (Reference) | ||
| 65+ | ||||||
| 1. SES lowest 20% | 1860 | 1.26 (1.19-1.34) | 1094 | 1.13 (1.04-1.22) | ||
| 2 | 2564 | 1.21 (1.15-1.28) | 1600 | 1.16 (1.08-1.24) | ||
| 3 | 2916 | 1.16 (1.10-1.22) | 1776 | 1.08 (1.01-1.15) | ||
| 4 | 3091 | 1.06 (1.01-1.12) | 1933 | 1.03 (0.97-1.10) | ||
| 5. SES highest 20% | 3116 | 1.00 (Reference) | 1994 | 1.00 (Reference) | ||
| Marital status at diagnosis | <.01 | <.01 | ||||
| Nonmarried (never married and previously married) | ||||||
| 1. SES lowest 20% | 1557 | 1.28 (1.19-1.38) | 942 | 1.17 (1.06-1.28) | ||
| 2 | 1683 | 1.17 (1.09-1.25) | 1082 | 1.13 (1.04-1.24) | ||
| 3 | 1807 | 1.06 (1.00-1.14) | 1139 | 1.03 (0.94-1.12) | ||
| 4 | 1842 | 1.01 (0.95-1.08) | 1180 | 1.00 (0.92-1.09) | ||
| 5. SES highest 20% | 1654 | 1.00 (Reference) | 1072 | 1.00 (Reference) | ||
| Married | ||||||
| 1. SES lowest 20% | 1436 | 1.34 (1.25-1.44) | 910 | 1.26 (1.16-1.38) | ||
| 2 | 2108 | 1.30 (1.23-1.38) | 1393 | 1.29 (1.20-1.39) | ||
| 3 | 2367 | 1.23 (1.16-1.30) | 1517 | 1.18 (1.10-1.26) | ||
| 4 | 2535 | 1.13 (1.07-1.20) | 1641 | 1.10 (1.03-1.18) | ||
| 5. SES highest 20% | 2776 | 1.00 (Reference) | 1817 | 1.00 (Reference) | ||
All Cox models were adjusted for age at diagnosis (continuous), sex, race/ethnicity, presence of B symptoms, nodal status, urbanicity, NCI-designated hospital, and first course (chemotherapy and radiation therapy) of treatment; stage at diagnosis (localized, regional, advanced, and unknown) was included as a stratifying variable for analyses by period of diagnosis and age group at diagnosis.
Discussion
In 33 032 patients diagnosed with DLBCL in California, mortality decreased by 30% from 1988 to 2000 to 2001 to 2009. Our findings are consistent with several clinical and population-based SEER studies reporting improved DLBCL survival,7,8 which is probably most attributable to the introduction of first-line rituximab into conventional chemotherapy. Although survival improved between the 2 time periods for all patient subgroups defined by age, sex, stage at diagnosis, race/ethnicity, and neighborhood SES, disparities in survival persisted. The magnitude of the neighborhood SES survival disparity was more pronounced in the modern treatment era after the emergence of rituximab, and in younger patients who otherwise have better DLBCL prognostic features.
Previous studies have found African-American DLBCL patients to have risks of death 10% to 20% higher than those for non-Hispanic whites,7,11-13 with survival differences persisting after the introduction of rituximab.7,11,12 Specifically, Komrokji reported that the median overall survival after diagnosis of DLBCL was 47 months in white patients vs 29 months in African-American patients from 2000 to 2004.7 In our study, we found elevated mortality risks in African Americans, Hispanics, and Asians/Pacific Islanders, compared with non-Hispanic whites, after controlling for factors besides neighborhood SES. However, after adjustment for neighborhood SES, racial/ethnic survival differences were attenuated, a finding consistent with 2 prior NHL survival analyses.32,33 In addition, our study showed that nonmarried patients experienced elevated mortality compared with married patients, an observation consistent with previous findings for many cancers including NHL.34-36 The most likely explanation is that married patients have stronger social support from spouses,34 which results in better adherence with prescribed treatments37 and less psychological difficulties,38 than nonmarried cancer patients. Our finding of the more marked inverse association between SES and mortality in married DLBCL patients may result from differences in insurance status between married and nonmarried DLBCL patients in our study. In a sensitivity analysis of pre-Medicare eligible patients (<65 years of age) diagnosed after 2001, we found that married patients were more likely to have access to private/military insurance; once insurance status was considered formally in the sensitivity analysis, all-cause and DLBCL-specific mortality was similar across levels of neighborhood SES (data not shown).
To our knowledge, this is the first report to demonstrate a significant neighborhood SES disparity in DLBCL mortality before and after the introduction of rituximab. Determinants relating to the patient, the tumor, and the health care system may contribute to this disparity.19 Later stage at diagnosis is one tumor factor that might mediate this disparity39-42 because of better access to care among higher SES communities. However, it seems unlikely that the disparity we observed could be explained only by stage at diagnosis, given that the association between neighborhood SES and mortality remained after stratification by stage at diagnosis.
Of health care factors that could impact cancer mortality,43 health insurance and access to care are likely to contribute to the observed disparity. Low SES individuals are less likely to have insurance coverage, or, even with coverage, are less likely to be able to pay high out-of-pocket costs for treatments. Because of insurance or other access factors, low SES individuals may also be less likely to obtain high-quality treatment and follow-up care,15,19 and more likely to have increased mortality even within the same stage at diagnosis.32,44,45 In the state of California, 43% of the population aged 18 to 65 years was uninsured or underinsured,46 while 97% of the population older than 65 years was covered by Medicare.47,48 In our study, we found that younger DLBCL patients residing in the lowest SES neighborhoods (those who are most likely to have insufficient insurance46 or who might have had poorer access to care) had 50% increased mortality risk, a higher mortality risk than found in older patients. We also observed increased mortality in underinsured DLBCL patients under the age of 65 years. Therefore, this study suggests that insufficient health insurance coverage for DLBCL patients from low SES neighborhoods may limit health care which, in turn, leads to poor survival outcomes. Our findings lend support to health care reform strategies implemented in the past few years, such as the Affordable Care Act, that aim to increase health care access and thereby alleviate health disparities by socioeconomic factors.49
Beyond coverage of health insurance, financial burden brought by health care–related cost may worsen the survival differences. Although considered cost-effective,50,51 adding rituximab to conventional chemotherapy amplifies general and total costs in DLBCL patients.52,53 Rising costs may place vulnerable populations subject to choices between cancer care and other necessities.46 Consistent with the literature on SES and general health outcomes,54 we found that, for DLBCL, the survival disparity across neighborhood SES persisted after controlling for source of health insurance for younger patients diagnosed after 2001. We also noted that the elevated overall mortality risk of patients in low vs high SES neighborhoods was more pronounced after the introduction of rituximab, whereas this pattern was not seen for lymphoma-specific mortality. These findings indicate that in the modern treatment era, disadvantaged populations are more likely to die of complications other than their cancer. Health care after cancer requires sophisticated management, including complicated drug regimens, general care, regular monitoring, as well as ongoing support of disease management.19,55,56 In the context of increased overall cost of living after diagnosis of DLBCL, disparities in maintaining general health care and self-management may further exacerbate poor outcomes among patients living in low SES neighborhoods.
Our study had the distinct strength of a population-based design with sufficient statistical power to detect variations in DLBCL survival across neighborhood SES and race/ethnicity with respect to clinical advances. The survival improvement with the use of rituximab reported in our and a previous SEER cancer registry study7 is generally lower than results from clinical-based studies.57-59 Unlike clinical studies, our large and diverse California population was not subject to predefined inclusion criteria or treated in specific hospitals/centers; thus, the results of our analyses are generalizable to the larger DLBCL patient population. Furthermore, population-based cancer registries have low levels of pathologic misclassification of DLBCL patients; 77% of cancer registry DLBCL diagnoses were confirmed by expert pathology re-review60 and an even higher concordance (87%) was demonstrated by the International Lymphoma Study group.2 Also, DLBCL classification has good reliability, as agreement of registry- and coder-assigned ICD-O-3 codes for DLBCL ranged from 84% to 89% for the period of 1988 to 2000.61
Although we were able to adjust outcomes with available demographic and clinical characteristics, a limitation of the current analysis is the lack of clinical information on DLBCL-specific measurements, such as serum LDH, performance status, treatment details beyond first course of therapy (including receipt of rituximab, which was recorded with the use of chemotherapy over the period of our study), and treatment of relapse, which may have confounded the association of neighborhood SES and DLBCL survival. In addition, recent observations of the impact of DLBCL cell of origin by gene expression studies62 support the concept that tumor-related factors are important in disease prognosis. However, information on DLBCL cell of origin is not available in the cancer registry. Also, information on primary source of payment was incomplete for 40% of patients diagnosed before 2001; therefore, we were unable to perform analysis of the impact of health insurance coverage on survival prior to the modern treatment era. Another limitation of the study is lack of an individual level of measurement of patient SES, which is not collected by cancer registries. However, studies have reported that neighborhood SES may augment, and not simply approximate, individual-level socioeconomic data63 by influencing health outcomes through the social (eg, social support), physical (eg, pollution), and built (eg, availability of health services) environments of the neighborhood.64,65 Our multifaceted, comprehensive measure of neighborhood SES incorporated several domains of education, income, employment, and cost of living that capture various elements of the socioeconomic environment. This neighborhood measurement has been used previously to demonstrate SES gradients in NHL survival.18,63,66
Prospective studies are needed to evaluate SES in association with existing prognostic factors67 in individual DLBCL patients so that those with lower SES can be identified prospectively in practice. This is critical to translating our observations into better outcomes, and would allow both an understanding of the true nature of the disparity in individual patients and the development of innovative strategies to extend the improvements in DLBCL survival with available therapies to all.
In summary, although DLBCL mortality has decreased remarkably, patients living in low SES neighborhoods in California experienced considerably increased mortality risk. Although we did not find racial/ethnic disparities in DLBCL survival after consideration for neighborhood SES, we found that neighborhood SES mortality disparities were more pronounced in younger patients, married patients, and in the modern treatment era. A sensitivity analysis suggests that insurance status could explain the mortality difference of neighborhood SES between married and nonmarried patients in our larger study population. Our findings suggest that inadequate insurance coverage with additional financial burden due to modern treatments may be associated with increased mortality after DLBCL for patients living in low SES neighborhoods. Future studies should seek to understand the widening of SES disparities and monitor how changes in the US health care system may contribute to reducing these disparities.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885 and the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute (NCI) at the National Institutes of Health (NIH) under contract HHSN2612010000140C awarded to the Cancer Prevention Institute of California (L.T., C.A.C., S.L.G., T.H.M.K.) and the Stanford Cancer Institute (C.A.C., S.L.G., and T.H.M.K.). J.M.F. did not receive financial support for this project. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885, the SEER Program of the NCI at the NIH under contract HHSN2612010000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute, and the Centers for Disease Control and Prevention (CDC) National Program of Cancer Registries, under agreement #1U58 DP000807-01 awarded to the Public Health Institute.
The ideas and opinions expressed herein are those of the authors and endorsement by the State of California, Department of Health Services, the NCI at the NIH, and the CDC or their contractors and subcontractors is not intended nor should be inferred.
Authorship
Contribution: L.T. and T.H.M.K. had full access to all of the data in the study, performed statistical analysis, and drafted the manuscript; and L.T., J.M.F., C.A.C., S.L.G., and T.H.M.K. designed research, interpreted data, critically revised the manuscript, and reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Li Tao, Cancer Prevention Institute of California, 2201 Walnut Ave, Suite 300, Fremont, CA 94538; e-mail: li.tao@cpic.org.
References
Author notes
L.T. and J.M.F. contributed equally to this study.