Key Points
TTP is inferior in patients with advanced-stage NLPHL compared with CHL.
Spleen involvement is associated with an increased risk of secondary aggressive lymphoma in patients treated with ABVD-like chemotherapy.
Abstract
Due to disease rarity, there is limited information regarding the optimal therapy and outcome for patients with advanced-stage nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL). Forty-two patients with NLPHL by the Revised European-American Lymphoma/World Health Organization classification with advanced-stage disease were identified and paired 1:2 with a matched control with classical Hodgkin lymphoma (CHL) matched by age, gender, stage, decade of diagnosis, and treatment received. The median follow-up was 11.3 years (range, 1.9 to 35.5 years) for NLPHL patients and 10.7 years (range, 1.6 to 26.3 years) for CHL patients. The majority received doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD)–like chemotherapy. Although the 10-year overall survival (OS) (P = .579) and HL freedom from treatment failure (HL-FFTF) were similar between NLPHL and CHL patients (75% vs 73%; P = .610), the time to progression (TTP), which also includes the development of secondary aggressive lymphoma, was inferior in NLPHL (10-year, 63% vs 73%; P = .040). Splenic involvement was associated with an inferior 10-year TTP in patients treated with ABVD (48% vs 71%; P = .049) and an increased cumulative incidence of secondary aggressive lymphoma (P = .014) providing a rationale for further evaluation of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) with rituximab in NLPHL.
Introduction
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) is a rare Hodgkin lymphoma (HL) subtype, representing only 5% of all cases.1 It is distinguished from classical HL (CHL) by the presence of large malignant cells known as lymphocyte-predominant (LP) cells, sometimes referred to as “popcorn” cells because of their lobulated appearance. In contrast to the Hodgkin-Reed-Sternberg (HRS) cells of CHL, the LP cells retain a B-cell phenotype with expression of CD20 and CD79a. The large retrospective review performed by the European Task Force on Lymphoma (ETFL) demonstrated that expert review by an experienced hematopathologist that incorporates both morphologic and immunophenotypic criteria is mandatory for the diagnosis of NLPHL.2 In this comprehensive analysis, just over half of the submitted cases were confirmed to be NLPHL, which brings into question the validity of older studies before the availability of immunophenotyping.
Clinically, patients with NLPHL typically present with limited-stage disease with involvement of peripheral node sites, and only 20% present with advanced-stage NLPHL at diagnosis. Unlike CHL, there is a tendency for multiple and late relapses. Furthermore, secondary aggressive non-HL (NHL), most commonly diffuse large B-cell lymphoma (DLBCL), has been noted to occur at a higher frequency in NLPHL.3-6 Whether this represents a true transformation from underlying NLPHL is a matter of debate. It may be the result of a common precursor cell or it may be the result of concurrent NLPHL and DLBCL at the time of diagnosis. There has been a wide range of rates of transformation reported across studies (0.9% to 14%)1,3-5 which may reflect multiple factors, including a lack of biopsy at disease relapse, variable risk profiles, absence of expert pathology review, and short follow-up in some studies, especially because this event has been reported to occur up to 20 years after diagnosis.4
Given the rarity of this disease, there is limited information regarding the optimal management of NLPHL, and this is particularly evident for advanced-stage disease. At the British Columbia Cancer Agency (BCCA), patients with HL of all types are similarly treated. Thus, the purpose of this study was to evaluate the outcome of patients with advanced-stage NLPHL compared with a cohort of matched historical controls with CHL to highlight the distinct natural history of this rare HL subtype.
Patients and methods
The BCCA Lymphoid Cancer database was searched to identify all patients age ≥12 years with advanced-stage NLPHL (stage III or IV disease or stage II with B symptoms and/or bulky disease) who were diagnosed between 1970 and 2011. Patients diagnosed before the routine use immunophenotyping used in the Revised European-American Lymphoma/World Health Organization classification7 were subjected to pathologic re-review by an expert BCCA hematopathologist (B.S.). The remaining cases had previously been centrally reviewed at the BCCA.
Clinical information was collected at the time of the diagnosis of NLPHL, including splenic involvement identified either by splenectomy at the time of staging laparotomy or by computed tomography (CT) imaging in the more modern treatment era (defined as splenic nodules or splenomegaly that resolves posttreatment). None of the patients had a staging positron emission tomography scan. In addition to standard doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) chemotherapy, other anthracycline-based treatment regimens included mechlorethamine, vincristine, procarbazine, and prednisone/doxorubicin, bleomycin, and vinblastine (MOPP/ABV); vincristine, doxorubicin, bleomycin, etoposide, and prednisone (ODBEP); and vinblastine, etoposide, cyclophosphamide, doxorubicin, bleomycin, vincristine, and prednisone (VECABOP), all of which were considered to be ABVD-like and have been previously shown to have equivalent efficacy.8,9 Prior to 1980, patients received MOPP chemotherapy or extended field radiotherapy (RT).
For each patient with NLPHL, two matched controls diagnosed with CHL were identified with blinding for outcome and matching by age (12-30, 31-45, 46-60, >60 years), gender, stage, decade of diagnosis (1970-1980, 1981-1990, 1991-2000, 2001-present), and chemotherapy received. This study was approved by the Research Ethics Board at the BCCA.
Statistical analysis
HL freedom from treatment failure (HL-FFTF) was measured from the date of pathologic diagnosis of HL to the date of relapse and/or progression or death as a result of HL or acute treatment toxicity. Time to progression (TTP) was measured from the date of diagnosis to the date of relapse and/or progression or death as a result of any lymphoma (including HL or development of a secondary NHL) or death as a result of acute treatment toxicity.10 Overall survival (OS) was measured from the date of diagnosis to the date of last follow-up or death from any cause. By using a competing risk analysis, the cumulative incidence of secondary aggressive NHL (time to transformation) was calculated from the date of diagnosis of NLPHL to the date of transformation to aggressive lymphoma in which deaths as a result of unrelated causes were treated as competing events. Baseline characteristics were compared by using the χ2 test. The Kaplan-Meier method was used to calculate survival and comparisons were made by using the log-rank test.11 All statistical analyses were performed by using SPSS, versions 11 and 14 (SPSS, Chicago, IL), and the cmprsk package of R software, version 2.14.2., was used for the competing risk analysis using Gray’s test.
Results
Baseline characteristics and primary therapy of patients with advanced-stage NLPHL vs matched controls with advanced-stage CHL
In total, 72 patients with advanced-stage NLPHL were identified, of whom 30 were excluded: 11 had concurrent DLBCL at diagnosis (composite, n = 7; discordant, n = 3) or were identified as a having a gray-zone lymphoma (n = 1); 6 had CHL (2 lymphocyte rich, 1 mixed cellularity, 1 lymphocyte depleted, 2 not otherwise specified); 2 had NHL; 1 elderly patient did not receive any therapy and was lost to follow-up; and 10 older patients for whom pathologic re-review was not possible because paraffin-embedded tissue was not available for immunophenotyping. For each of the 42 remaining patients with NLPHL, two matched CHL controls were identified as described above.
For the NLPHL patients, the median age was 37 years (range, 12 to 75 years) and the majority were male (71%) with a good performance status (PS ≤1; 90%). All had low-risk disease by the IPS (Table 1).12 Most patients had stage III disease (90%), and B symptoms were present in only 9 patients (21%). The median mass size was 4 cm (range, 1 to 9 cm), and none of the patients had bulky disease (mass ≥10 cm). Spleen involvement was present in 14 patients (33%): 5 identified by splenectomy at the time of staging laparotomy, 8 by CT imaging (7 splenic nodules, 1 splenomegaly), and 1 through documentation in the clinical notes. Most patients received standard ABVD (n = 27; 64%) or ABVD equivalent (ABVD-like) chemotherapy (MOPP-ABVD, n = 1; MOPP/ABV, n = 5; ODBEP, n = 18 ; VECABOP, n = 213 ) (total ABVD-like, n = 36; 85%), none of whom received consolidation RT. A minority received MOPP alone (n = 4) with or without RT and two patients diagnosed in the earliest treatment era were treated with extended field RT alone.
Baseline characteristics of patients with advanced-stage NLPHL and matched controls with advanced-stage CHL
| Characteristic . | NLPHL (n = 42) . | CHL (n = 84)* . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| Follow-up, years | ||||
| Median | 11.3 | 10.7 | ||
| Range | 1.9-35.5 | 1.6-26.3 | ||
| Male sex | 30 | 71 | 58 | 69 |
| Age, years | ||||
| Median | 37 | 37 | ||
| Range | 12-75 | 12-78 | ||
| ≥45 | 17 | 40.5 | 31 | 37 |
| Stage | ||||
| II | 2 | 5 | 4 | 5 |
| III | 37 | 90 | 76 | 90 |
| IV | 2 | 5 | 4 | 5 |
| PS ≥2 | 4 | 9.5 | 8 | 9.5 |
| B symptoms | 9 | 21 | 18 | 21 |
| Extranodal disease | 2 | 5 | 8 | 9.5 |
| Spleen involvement | 14 | 33 | 33 | 39 |
| Bulky mass ≥10 cm | 0 | 0 | ||
| Mass ≥5 cm | 13 | 31 | 38 | 45 |
| IPS ≥4 | 0 | 5 | 6 | |
| Elevated LDH | 3 | 7 | 16 | 19 |
| Treatment | ||||
| ABVD-(like) chemotherapy | 36 | 86 | 74 | 88 |
| MOPP ± radiation | 4 | 9.5 | 6 | 7 |
| Radiation alone | 2 | 5 | 4 | 5 |
| Characteristic . | NLPHL (n = 42) . | CHL (n = 84)* . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| Follow-up, years | ||||
| Median | 11.3 | 10.7 | ||
| Range | 1.9-35.5 | 1.6-26.3 | ||
| Male sex | 30 | 71 | 58 | 69 |
| Age, years | ||||
| Median | 37 | 37 | ||
| Range | 12-75 | 12-78 | ||
| ≥45 | 17 | 40.5 | 31 | 37 |
| Stage | ||||
| II | 2 | 5 | 4 | 5 |
| III | 37 | 90 | 76 | 90 |
| IV | 2 | 5 | 4 | 5 |
| PS ≥2 | 4 | 9.5 | 8 | 9.5 |
| B symptoms | 9 | 21 | 18 | 21 |
| Extranodal disease | 2 | 5 | 8 | 9.5 |
| Spleen involvement | 14 | 33 | 33 | 39 |
| Bulky mass ≥10 cm | 0 | 0 | ||
| Mass ≥5 cm | 13 | 31 | 38 | 45 |
| IPS ≥4 | 0 | 5 | 6 | |
| Elevated LDH | 3 | 7 | 16 | 19 |
| Treatment | ||||
| ABVD-(like) chemotherapy | 36 | 86 | 74 | 88 |
| MOPP ± radiation | 4 | 9.5 | 6 | 7 |
| Radiation alone | 2 | 5 | 4 | 5 |
There were missing values for mass size: NLPHL n = 3, CHL n = 4; spleen involvement: NLPHL n = 1; PS: NLPHL n = 1, CHL n = 2; elevated LDH: NLPHL n = 7, CHL n = 18; IPS score: NLPHL n = 5; CHL n = 11.
IPS, International Prognostic Score; LDH, lactate dehydrogenase.
Matching was performed for 42 NLPHL patients.
For the matched control analysis, 84 patients with CHL were identified, most of whom had nodular sclerosis CHL (n = 67; 80%), and the remaining patients had mixed cellularity (n = 9), lymphocyte-rich (n = 2), lymphocyte-depleted (n = 1), or CHL not otherwise specified (n = 5) subtypes. Eight of 84 matched controls deviated from the matching schema: 1 control received MOPP/ABVD instead of the MOPP chemotherapy that the NLPHL patient received, 5 were identified in the preceding (n = 2) or following (n = 3) decade, and 2 were from the next age group. The groups were equivalent for the matched baseline characteristics. Similarly, for unmatched characteristics, there were no differences between the groups (PS P = 1.000, B symptoms P = 1.000, IPS ≥4 P = .259, elevated LDH P = .259, extranodal involvement P = .351, mass size ≥5 cm P = .124, and spleen involvement P = .608). Spleen involvement was documented in 33 patients with CHL, 9 by splenectomy at the time of staging laparotomy and the remainder on CT imaging (22 had splenic nodules, 2 had splenomegaly).
Outcome analysis: NLPHL vs matched CHL controls
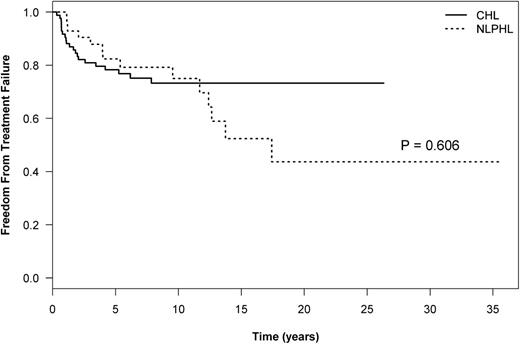
The median follow-up for NLPHL and CHL patients was 11.3 years (range, 1.9 to 35.5 years) and 10.7 years (range, 1.6 to 26.3), respectively. The 10-year HL-FFTF, which reflects only relapses from HL, was similar between patients with NLPHL or CHL (75% vs 73%, respectively; P = .610) (Figure 1; Table 2). However, late relapses of ≥5 years were more common in patients with NLPHL than in those with CHL (21% [n = 9] vs 2.4% [n = 2]; P = .001), representing 45% and 10% of all relapses, respectively, and a plateau in the HL-FFTF curve is not apparent in NLPHL (Figure 1). Transformation to an aggressive NHL occurred exclusively in NLPHL (Table 2). By using a competing risk analysis, the cumulative risk of transformation at 15 years was 24%. There was a trend to a worse OS in ever-transformed vs never-transformed patients (10-year OS, 92% vs 56%; P = .173). The median time to transformation in NLPHL patients was 5.45 years (range, 0.3 to 20.3 years). This also translated into an inferior TTP (P = .040) (Figure 2;,Table 2) in patients with NLPHL compared to those with CHL, which encompasses relapses from both HL and aggressive NHL. Similar results were obtained if the TTP analysis was confined to only patients with NLPHL and CHL receiving ABVD-like chemotherapy (P = .041).
Outcome of patients with advanced-stage NLPHL compared with matched controls with advanced-stage CHL
| . | HL subtype . | Survival (%) . | P . | ||
|---|---|---|---|---|---|
| 5-Year . | 10-Year . | 15-Year . | |||
| HL-FFTF | NLPHL | 82 | 75 | 52 | .610 |
| CHL | 78 | 73 | 73 | ||
| TTP | NLPHL | 72 | 63 | 44 | .040 |
| CHL | 78 | 73 | 73 | ||
| OS | NLPHL | 89 | 83.5 | 74 | .826 |
| CHL | 91 | 81 | 68 | ||
| TTT | NLPHL | 12 | 15 | 24 | .00018 |
| CHL | 0 | 0 | 0 | ||
| . | HL subtype . | Survival (%) . | P . | ||
|---|---|---|---|---|---|
| 5-Year . | 10-Year . | 15-Year . | |||
| HL-FFTF | NLPHL | 82 | 75 | 52 | .610 |
| CHL | 78 | 73 | 73 | ||
| TTP | NLPHL | 72 | 63 | 44 | .040 |
| CHL | 78 | 73 | 73 | ||
| OS | NLPHL | 89 | 83.5 | 74 | .826 |
| CHL | 91 | 81 | 68 | ||
| TTT | NLPHL | 12 | 15 | 24 | .00018 |
| CHL | 0 | 0 | 0 | ||
TTT, time to transformation.
The OS was similar between patients with NLPHL and those with CHL (P = .826) (Figure 3;,Table 2), but deaths as a result of HL were the predominant cause of mortality in the CHL group (47%) whereas for patients in the NLPHL group, deaths as a result of secondary aggressive NHL were a common cause of mortality (40%) (Table 3) (P = .021).
Cause of death in NLPHL and CHL patients
| Cause of death . | NLPHL (n = 10) . | CHL (n = 17) . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| HL | 1 | 10 | 8 | 47 |
| Aggressive NHL | 4 | 40 | 0 | |
| Secondary cancers | 1 | 10 | 3 | 18 |
| Cardiac | 4 | 40 | 6 | 35 |
| Cause of death . | NLPHL (n = 10) . | CHL (n = 17) . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| HL | 1 | 10 | 8 | 47 |
| Aggressive NHL | 4 | 40 | 0 | |
| Secondary cancers | 1 | 10 | 3 | 18 |
| Cardiac | 4 | 40 | 6 | 35 |
Risk factors for transformation and recurrent NLPHL in advanced-stage NLPHL
Among the 42 patients with NLPHL, 21 relapsed following initial therapy: 14 with NLPHL and 7 with aggressive NHL. One patient with pathologically confirmed NLPHL was clinically suspected to have aggressive NHL on the basis of clinical presentation (rapidly enlarging 20-cm abdominal mass, LDH 5 times the upper limit of normal).
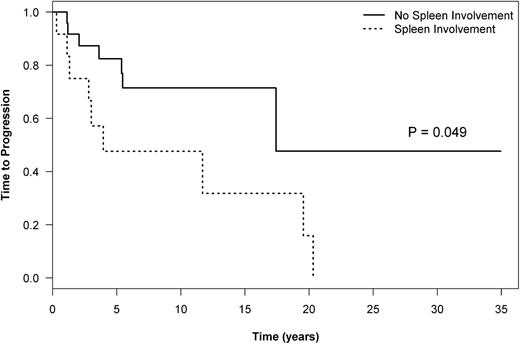
By using a competing risk analysis, spleen involvement at the time of diagnosis of NLPHL was associated with a significantly increased cumulative incidence of transformation (10-year cumulative incidence, 29% vs 7.8%; P = .019; Table 4). Similarly, considering only the patients who received ABVD-like treatment, the cumulative incidence of transformation at 10 years was 34% in the spleen-positive group compared with 9% in the spleen-negative group (P = .014). This was also reflected in an inferior TTP in patients with splenic involvement treated with ABVD-like regimens (10-year TTP, 48% vs 71%; P = .049, Figure 4). Interestingly, if only patients without spleen involvement are considered, the TTP is similar in patients with CHL and NLPHL who received ABVD-like treatment (10-year TTP, 74% vs 71%; P = .840).
P values for univariate analysis of risk factors for transformation to secondary aggressive NHL, HL-FFTF, and TTP
| Clinical feature . | TTT to secondary NHL . | HL-FFTF (relapse NLPHL) . | TTP (relapse all lymphoma) . | OS . |
|---|---|---|---|---|
| Male gender | .149 | .040 | .113 | .504 |
| Age ≥45 years | .703 | .466 | .469 | .865 |
| B symptoms | .412 | .688 | .239 | .043 |
| PS ≥2 | .289 | .0007 | .058 | .025 |
| Splenic disease | .019 | .760 | .001 | .521 |
| Mass ≥5 cm | .011 | .194 | .480 | .645 |
| Elevated LDH | .198 | .306 | .904 | .493 |
| ABVD-like | .755 | .147 | .744 | .728 |
| Clinical feature . | TTT to secondary NHL . | HL-FFTF (relapse NLPHL) . | TTP (relapse all lymphoma) . | OS . |
|---|---|---|---|---|
| Male gender | .149 | .040 | .113 | .504 |
| Age ≥45 years | .703 | .466 | .469 | .865 |
| B symptoms | .412 | .688 | .239 | .043 |
| PS ≥2 | .289 | .0007 | .058 | .025 |
| Splenic disease | .019 | .760 | .001 | .521 |
| Mass ≥5 cm | .011 | .194 | .480 | .645 |
| Elevated LDH | .198 | .306 | .904 | .493 |
| ABVD-like | .755 | .147 | .744 | .728 |
TTP in patients treated with ABVD by spleen involvement at diagnosis of NLPHL.
A large tumor mass (≥5 cm) at initial presentation was also identified as a risk factor for transformation (P = .011), and the presence of B symptoms was associated with an inferior OS (Table 4). Poor PS was associated with an inferior HL-FFTF and OS, and a trend was observed for TTP; however, this finding was present in only a small group of patients (Table 1). Male gender was associated with an inferior HL-FFTF, and a trend toward an inferior HL-FFTF was observed in patients receiving therapy that did not include ABVD-like chemotherapy (10-year HL-FFTF, 78.5% vs 67%; P = .147) (Table 4).
Therapy for relapsed NLPHL and secondary aggressive NHL
Of the 14 patients with relapsed NLPHL, 7 had advanced-stage disease, 6 had limited-stage disease, and 1 had unknown disease status. As described, 1 patient was treated as having aggressive lymphoma because of a suggestive clinical presentation. For the remaining 13 patients, therapy was heterogeneous: 4 patients received second-line chemotherapy with gemcitabine, dexamethasone, and cisplatin (GDP) (n = 3) or MOPP (n = 1) followed by high-dose chemotherapy and autologous stem cell transplant; 4 received ABVD-like chemotherapy; 2 received RT alone; and 1 each received rituximab, cyclophosphamide, vincristine, and prednisolone (R-CVP) chemotherapy, rituximab alone, or lomustine. Eight patients subsequently relapsed with NLPHL, one of whom had a third relapse of aggressive NHL 7 years after the initial diagnosis; thus, overall, almost 60% of patients had a subsequent lymphoma relapse.
Nine patients ultimately developed a secondary aggressive NHL, 4 of whom the pathology showed DLBCL, 2 resembled T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL-like), and 1 case showed DLBCL with areas resembling THRLBCL and a subsequent relapse was consistent with THRLBCL-like histology. In one patient, a fine-needle aspirate demonstrated aggressive NHL; however, full subclassification was not possible because of limited pathologic material, and the remaining patient was presumed to have transformed disease, given an aggressive course as described above. In this group, 3 patients received high-dose chemotherapy and autologous stem cell transplant and 5 patients received cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) –like chemotherapy with (n = 4) or without (n = 1) rituximab and with etoposide substitution if the anthracycline maximal dose was reached. One patient received palliative therapy only because of a poor PS. Four patients subsequently died of aggressive lymphoma. However, for the surviving patients, there were no subsequent lymphoma relapses.
Discussion
Patients with NLPHL infrequently present with advanced-stage disease and as a result, there is limited information regarding outcome, risk of transformation and optimal therapy. Interpretation of older studies remains challenging because they often lack pathologic re-review using modern diagnostic criteria or follow-up is too short given the propensity for late relapse.
We sought to compare the outcome of patients with advanced-stage NLPHL primarily treated with ABVD-like chemotherapy with a matched cohort of patients with advanced-stage CHL in an effort to highlight differences in the natural history. Although relapses from HL were similar in both cohorts, late relapses were more common in the NLPHL cohort, confirming observations in other series.1 Male gender was associated with an increased risk of NLPHL relapse. A recent study from the German Hodgkin Lymphoma Study Group (GHSG) also found that male gender was associated with an increased risk of relapse and/or progression in NLPHL patients in addition to low albumin.14 Further, variant histopathologic growth pattern, as previously defined by Fan and colleagues as the presence of LP cells outside of B-cell nodules or reduced numbers of non-neoplastic B cells within the nodules, was associated with advanced-stage disease and an inferior progression-free survival (PFS) compared with typical NLPHL histology, but whether this reflects relapse from NLPHL or the development of secondary aggressive lymphoma is unknown.15
Not surprisingly, in our analysis, the development of secondary aggressive lymphoma occurred exclusively in NLPHL with a cumulative risk at 15 years of 24%, but this also translated into an inferior TTP compared with CHL, an end point which comprises all lymphoma relapses (P = .040). Considering only patients treated with ABVD-like therapy, by 10 years, approximately 40% have relapsed with either NLPHL or secondary aggressive lymphoma. Those with splenic involvement at diagnosis have a 10-year TTP of only 48% compared with 71% for those without splenic involvement, suggesting that ABVD may be inadequate in this subgroup, possibly because of the increased risk of harboring occult aggressive lymphoma. Interestingly, in 3 patients, the spleen was removed during staging laparotomy at the time of the initial diagnosis of NLPHL and thus, the sole reason cannot be the presence of occult aggressive disease in the spleen. The cumulative incidence of aggressive lymphoma in our study is higher than that reported in the literature. This probably reflects our focus on patients with advanced-stage disease in addition to mature follow-up (median, >11 years), because this event can occur decades after the original diagnosis in addition to pathologic review at the time of lymphoma relapse. Notably, a trend to a worse OS was observed in patients who developed transformed lymphoma.
There have been very few prior studies evaluating the outcome of patients with advanced-stage NLPHL. The largest study reported by the GHSG described 82 patients with advanced-stage NLPHL (stage IIB with risk factors and stage III to IV) who were evaluated as part of a larger retrospective study comparing patients with all stages of NLPHL or CHL treated on GHSG trials from 1988 to 2002.15 Treatments were heterogeneous and included cyclophosphamide, vincristine, procarbazine, and prednisone (COPP)-ABVD + RT, COPP-ABV-ifosfamide, methotrexate, etoposide, and prednisolone (IMEP) + RT, and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, and procarbazine (BEACOPP) baseline or escalated (or combined) ± RT. In that study, the median follow-up was only 4.2 years, limited-stage and advanced-stage patients were analyzed together for many of the outcome comparisons, and the type of lymphoma relapse was not reported. The FFTF (events were relapse or progression, death, and lack of a complete response) was similar in patients with advanced-stage NLPHL and CHL (4.2-year HL-FFTF, 77% vs 75%). This end point is best compared with our TTP definition in which we demonstrated a similar 25% lymphoma relapse rate in both HL subtypes at 5 years (Table 2); however, with longer follow-up, the TTP curves diverge because of late lymphoma relapses, some of which represent secondary aggressive lymphoma (Figure 2). In the GHSG study, the OS was found to be superior in NLPHL; however, patients with advanced-stage disease were not evaluated separately.
The ETFL evaluated 44 patients with advanced-stage NLPHL who were mostly treated in the 1980s; thus, almost half the patients received MOPP alone. With a median follow-up of 6.8 years for patients of all stages, the 8-year HL-FFTF was 62% and 24% in stage III (n = 31) and stage IV (n = 13) patients, respectively, with corresponding OS estimates of 94% and 41%.1 Patients with stage IV disease are exceedingly rare and represent only 5% of all patients with advanced-stage disease in our study. In the ETFL analysis, almost half the patients with stage IV disease had liver involvement on clinical grounds but confirmatory biopsies were not routinely undertaken. Given the rarity of liver involvement with NLPHL in our series and most other series, it seems likely that the EFTL patients harbored aggressive disease that was not optimally treated with HL-directed therapies. Again, the type of lymphoma relapse was not reported.
There is continued debate regarding the optimal chemotherapy in NLPHL and whether it should be treated similarly to CHL. This lack of consensus is highlighted in the diversity of choices recommended in both the European Society for Medical Oncology (ESMO) and the National Comprehensive Cancer Network (NCCN) guidelines for advanced-stage disease.16,17 The ESMO guidelines for NLPHL tend to follow recommendations for CHL with either ABVD or escalated BEACOPP as treatment options. In contrast, the NCCN recommendations are diverse and include ABVD as well as cyclophosphamide, vincristine, and prednisone (CVP); CHOP; and dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (DA-EPOCH). Further, noncurative approaches such as use of single-agent rituximab, palliative RT, or observation have also been suggested for those with asymptomatic disease.16
Some studies have suggested that alkylator-based chemotherapy may be superior in NLPHL,18 possibly reflecting a bias that NLPHL is best managed similarly to indolent NHL. A pooled study of 37 patients with advanced-stage NLPHL treated on Cancer and Leukemia Group B (CALGB) studies and at the Dana-Farber Cancer Center/Joint Center for Radiation Therapy (DFCI/JCRT) described a 75% failure rate in patients treated with ABVD or etoposide, vinblastine, and doxorubicin (EVA) compared with only 32% in patients treated with MOPP or MOPP/ABVD.18 However, interpretation is challenging because of the small number of patients—only 12 patients were treated with either ABVD or EVA (which subsequently was shown to be inferior to ABVD19 )—as well as the inclusion of patients who had relapsed following RT (which may represent a different risk group). Furthermore, the patients included in that retrospective analysis were diagnosed in an era prior to routine immunophenotyping and thus, the diagnosis of NLPHL may be unreliable.
At the BCCA, the treatment approaches for NLPHL and CHL have been similar, and thus, in recent years, the large majority of patients have been treated with ABVD-like chemotherapy. Gene expression studies support a close relatedness of NLPHL with CHL but also with THRLBCL, highlighting its unique biology.20 A more recent study by Hartmann et al21 compared the gene expression profile of microdissected tumor cells from NLPHL, THRLBCL-like NLPHL (defined as having at least one typical NLPHL nodule), and THRLBCL and found significant molecular overlap between these entities. Pairwise supervised comparisons also demonstrated very few differentially expressed genes, and it is speculated that the composition of the microenvironment defines these entities, which may in turn reflect the host immune status.21 In our analysis, there was an excess of lymphoma relapses in NLPHL compared with CHL using ABVD-like chemotherapy which, in some instances, resembled THRLBCL. Further pathologic studies are needed to define which NLPHL patients may be better suited for aggressive lymphoma therapies.
Given that LP cells are strongly CD20 positive, rituximab has been evaluated in NLPHL. The results from a phase 2 study evaluating single-agent rituximab in 39 relapsed and newly diagnosed patients, half of which had advanced-stage disease, are now mature.20 With a high relapse rate observed with the standard treatment administered once per week for 4 weeks (5-year PFS, 39%), the protocol was amended to include maintenance rituximab every 6 months for 2 years. Although the latter was associated with an improved 5-year PFS of 58.9%, these results appear inferior to those achieved with combination chemotherapy, and this approach does not appear to be curative. Secondary transformation to aggressive B-cell lymphoma was noted in 9 (39%) of 23 of the relapsed patients, including both DLBCL and THRLBCL. Interestingly, 6 of 9 of these patients had infradiaphragmatic involvement at study entry, but splenic involvement was not documented. More recently, the MD Anderson Cancer Center (MDACC) evaluated the outcome of 15 NLPHL patients treated with rituximab plus CHOP (R-CHOP) chemotherapy (advanced stage, n = 11).22 With a median follow-up of 42 months, there have been no relapses or transformations. Although the follow-up is short and the number of patients small, these findings support further study of R-CHOP chemotherapy for patients with NLPHL, especially for those with splenic and perhaps infradiaphragmatic involvement at diagnosis. Longer follow-up will be necessary to determine whether R-CHOP can alter the natural history of NLPHL, including late NLPHL relapses, sufficiently to guide usage for all patients.
In summary, this analysis highlights the distinct disease behavior of NLPHL compared with CHL and the need for repeat biopsy at relapse as well as long-term surveillance. With mature follow-up, the risk of lymphoma relapse is higher in NLPHL than in CHL, and splenic involvement represents an inherent risk factor for the future development of transformed aggressive lymphoma. Our study provides a historical comparison of the outcome of advanced-stage NLPHL with primarily ABVD-like therapy and provides a rationale for further evaluation of CHOP with the addition of rituximab, given strong expression of CD20.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.X. participated in study design, chart review, data collection and manuscript writing; K.J.S. conceived of the study, participated in study design, data collection, statistical analysis, data interpretation, and manuscript writing; J.M.C. participated in study design; M.A.-M. participated in the chart review; A.L. performed the statistical analysis; R.D.G. and B.S. performed pathologic review; and all authors contributed to patient data and manuscript review and read and approved the final manuscript.
Conflict-of-interest disclosure: K.J.S., L.H.S., J.M.C., and R.D.G. have received research funding from Roche. The remaining authors declare no competing financial interests.
Correspondence: Kerry J. Savage, 600 West 10th Ave, Vancouver, BC, Canada V5Z 4E6; email: ksavage@bccancer.bc.ca.