Key Points
Eltrombopag/dexamethasone is a safe and effective combination for treating newly diagnosed ITP patients.
This treatment may prove useful in achieving lasting responses without additional immunosuppression in some patients.
Abstract
Immune thrombocytopenia (ITP) results from platelet destruction and production suppression. Eltrombopag belongs to a new class of thrombopoietin-mimetic drugs that raise platelet counts in ITP patients. We performed a single-arm study to assess the response to a single course of dexamethasone (40 mg by mouth, days 1-4) in combination with eltrombopag (50 mg, days 5-32) in 12 adults with newly diagnosed ITP in an outpatient setting. Median follow-up was 12.5 months. After therapy (day 33), 100% of patients achieved at least ≥30 × 109/L platelets. Four patients relapsed. Complete response at 6 months (platelets ≥100 × 109/L) was achieved in 50% of patients and response at 6 months (platelets ≥30 <100 × 109/L) was achieved in another 25%; relapse-free survival was 66.7% at 12 months (median response duration of 8.3 months). In conclusion, eltrombopag/dexamethasone is a feasible frontline therapy for ITP. This trial is registered at www.clinicaltrials.gov as NCT01652599.
Introduction
Immune thrombocytopenia (ITP) is a disease that results from autoimmune destruction of platelets and suppression of platelet production.1 A platelet count (Plt) <30 × 109/L generally indicates severe disease. Newly diagnosed ITP is considered as lasting up to 3 months, followed by persistent (3-12 months) and chronic disease (>12 months).2 Frontline therapy includes corticosteroids and intravenous immune γ-globulin (IVIG) or anti-D immunoglobulin. Prednisone at a dose of 1 to 2 mg/kg raises the Plt in 70% to 80% of patients; however, only a small portion will achieve a sustained remission.3 High-dose dexamethasone is another option; 40 mg/day (4 days) was initially effective in 85% of patients. Nevertheless, 50% relapsed within 6 months.4 Corticosteroids remain the standard of care, but high failure/relapse rates and considerable adverse effects from long-term use continue to stimulate the search for better treatments.3,5,6 We and others have reported sustained response rates ranging from 58% to 76% using rituximab plus dexamethasone as a frontline therapy.7-10 Eltrombopag is a thrombopoietin (TPO) nonpeptide mimetic that has been shown to raise the Plt in both continued long-term administration and in a repeated short-term administration in chronic ITP.11-13 Eltrombopag has never been used as frontline therapy in acute ITP; therefore, the purpose of this study was to assess its efficacy in this setting in combination with dexamethasone.
Study design
This open-label, single-arm study was performed on patients with newly diagnosed ITP from the Hospital Universitario “Dr José E. González” in Monterrey, Mexico, and Clínica Ruíz, in Puebla, México.14 Eligible patients were age 18 years or older, with bleeding manifestations according to the International Working Group assessment,15 a Plt of <30 × 109/L, and no previous treatment. Participants were excluded if they had an active infection; drug-associated thrombocytopenia; positive serology for HIV, hepatitis B, or hepatitis C; malignant diseases; or were pregnant. Cardiovascular risk factors were not exclusion criteria. Each institution’s ethics committee approved the study and patients gave written informed consent. This study was conducted in accordance with the Declaration of Helsinki. Our primary outcome was end-of-treatment (day 33) response rate. Secondary outcome measures included response at 6 months and relapse-free survival (RFS) at 6 and 12 months.
Treatment consisted of dexamethasone, 40 mg/day by mouth for 4 days (1-4), and eltrombopag, 50 mg by mouth once a day for 28 days (beginning at day 5). Instructions were provided to avoid calcium supplements and dairy products 4 hours before or after taking eltrombopag. Treatment was administered in an outpatient setting. A complete blood count was performed at baseline, on day 5, then weekly for 28 days, monthly until month 6, and every 3 months thereafter. Eltrombopag was suspended if platelets were ≥400 × 109/L. Response (R) at day 33 and complete response (CR) at day 33 were defined as an increase in platelets ≥30 <100 × 109/L and ≥100 × 109/L, respectively.2 R at 6 months (R2) and CR at 6 months (CR2) were determined when platelets reached ≥30 <100 × 109/L and ≥100 × 109/L, respectively.2 RFS was considered to be from the day of initial response until relapse (Plt <30 × 109/L). Duration of response (DOR) included the entire period of any responses achieved (CR or R). Treatment safety profiles and side effects were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. The Kaplan-Meier method was used to calculate probability of RFS; the analysis was performed with SPSS software version 20.0 for Mac.
Results and discussion
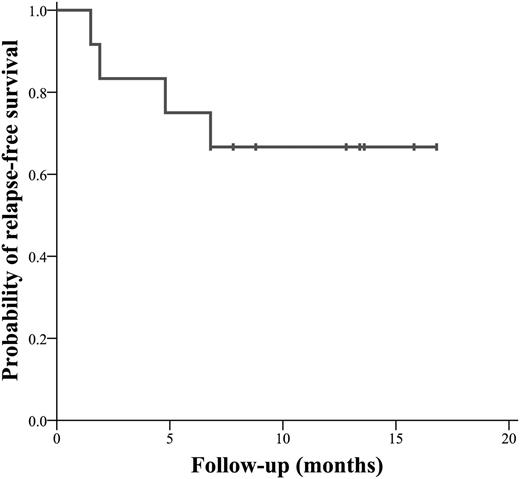
Twelve patients were enrolled between June 2012 and July 2013. Nine patients (75%) had grade 2 bleeding manifestations. Median Plt at diagnosis was 7 × 109/L (range 2-28). Median follow-up was 12.5 months (range 7-18) (Table 1). After dexamethasone treatment, 5 patients responded and 5 achieved CR. All patients achieved either R or CR by the time treatment with eltrombopag was complete (median 5 days, range 5-19). The CR rate was 83.3%, whereas the R rate was 16.7%. Patients who had a prompt response to dexamethasone (n = 5) also showed a faster and higher increase in Plt. Six patients had >400 × 109/L platelets the day eltrombopag was stopped (day 33). Four patients (33.3%) relapsed in a median time of 14.5 weeks (range 6.3-29.7); 2 regained CR and 1 regained R after treatment using dexamethasone/rituximab,9 whereas the remaining patient sought treatment elsewhere. R2 was achieved in 25%, whereas CR2 was achieved in 50% of patients. Probability of RFS was 66.7% at 12 months (Figure 1). Currently, 4 patients remain in CR and 4 in R. Best response was achieved in a median of 33 days (range 12-60) and median DOR was 8.3 months (range 1.5-16.8). Bleeding manifestations disappeared in all patients. No adverse events occurred, no data suggestive of myelofibrosis or venous thrombosis were observed, and the drug combination was well tolerated.
Baseline characteristics and results of 12 newly diagnosed ITP patients treated with a single course of high-dose dexamethasone and eltrombopag
| N . | Age, y/sex . | Type of bleeding . | Bleeding grade . | Time to PR/CR . | Day 5 . | Day 34 . | Month 3 . | Month 6 . | DOR, wk . | Relapse . | Follow-up, mo . | Current status . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38/F | He/Gu/Ep | 2 | –/5 | CR | CR | CR* | CR* | 6.5 | Yes | 18 | CR* |
| 2 | 49/F | Ec/Me | 2 | –/5 | CR | CR | PR | PR | 72 | — | 17 | PR |
| 3 | 40/F | Ec/Gu | 1 | –/5 | CR | CR | CR | CR | 68 | — | 16 | CR |
| 4 | 20/M | Ec | 2 | 12/26 | NR | CR | PR | PR | 58.5 | — | 14 | PR |
| 5 | 60/M | He | 2 | 19/26 | NR | PR | PR | PR | 57.6 | — | 14 | PR |
| 6 | 68/F | Pe/Gu/Re | 2 | –/5 | CR | CR | CR | CR | 55 | — | 13 | CR |
| 7 | 79/F | Pe/Gu/Ep | 2 | 5/19 | PR | CR | PR* | PR* | 8 | Yes | 12 | PR* |
| 8 | 40/F | Pe/Ec/Ep | 1 | 5/34 | PR | CR | PR | CR | 37.8 | — | 9 | CR |
| 9 | 80/M | Pe/Hem | 3 | –/5 | CR | CR | CR | CR* | 20.6 | Yes | 8 | CR* |
| 10 | 68/M | He | 2 | 5/19 | PR | CR | CR | CR | 33.5 | — | 8 | CR |
| 11 | 30/M | Pe/Ec/Ep | 2 | 5/26 | PR | CR | CR | CR | 29.4 | — | 7 | CR |
| 12 | 51/M | Pe/He | 2 | 5/12 | PR | PR | CR | CR | 29.4 | Yes | 7 | SD* |
| N . | Age, y/sex . | Type of bleeding . | Bleeding grade . | Time to PR/CR . | Day 5 . | Day 34 . | Month 3 . | Month 6 . | DOR, wk . | Relapse . | Follow-up, mo . | Current status . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38/F | He/Gu/Ep | 2 | –/5 | CR | CR | CR* | CR* | 6.5 | Yes | 18 | CR* |
| 2 | 49/F | Ec/Me | 2 | –/5 | CR | CR | PR | PR | 72 | — | 17 | PR |
| 3 | 40/F | Ec/Gu | 1 | –/5 | CR | CR | CR | CR | 68 | — | 16 | CR |
| 4 | 20/M | Ec | 2 | 12/26 | NR | CR | PR | PR | 58.5 | — | 14 | PR |
| 5 | 60/M | He | 2 | 19/26 | NR | PR | PR | PR | 57.6 | — | 14 | PR |
| 6 | 68/F | Pe/Gu/Re | 2 | –/5 | CR | CR | CR | CR | 55 | — | 13 | CR |
| 7 | 79/F | Pe/Gu/Ep | 2 | 5/19 | PR | CR | PR* | PR* | 8 | Yes | 12 | PR* |
| 8 | 40/F | Pe/Ec/Ep | 1 | 5/34 | PR | CR | PR | CR | 37.8 | — | 9 | CR |
| 9 | 80/M | Pe/Hem | 3 | –/5 | CR | CR | CR | CR* | 20.6 | Yes | 8 | CR* |
| 10 | 68/M | He | 2 | 5/19 | PR | CR | CR | CR | 33.5 | — | 8 | CR |
| 11 | 30/M | Pe/Ec/Ep | 2 | 5/26 | PR | CR | CR | CR | 29.4 | — | 7 | CR |
| 12 | 51/M | Pe/He | 2 | 5/12 | PR | PR | CR | CR | 29.4 | Yes | 7 | SD* |
CR, Plt ≥100 × 109/L; Ec, ecchymoses; Ep, epistaxis; F, female; Gu, gum; He, hematomas; Hem, hematuria; M, male, Me, menorrhagia; N, patient number; NR, no response; Pe, petechiae; PR, response was defined as platelets 30 to <100 × 109/L and doubling from baseline; Re, rectorrhagia; SD, stable disease.
Patients had previously relapsed. Results are shown after treatment with high-dose dexamethasone and low-dose rituximab.
Probability of relapse (thrombocytopenia) free survival in 12 patients with newly diagnosed ITP treated with a single course of eltrombopag and high-dose dexamethasone.
Probability of relapse (thrombocytopenia) free survival in 12 patients with newly diagnosed ITP treated with a single course of eltrombopag and high-dose dexamethasone.
This proof-of-concept trial suggests that high initial responses can be obtained with eltrombopag/dexamethasone: 5 patients obtained CR at day 5, whereas the remaining 7 improved after the addition of eltrombopag, increasing the CR rate to 83.3%. These responses appear at least comparable to other first-line strategies in acute ITP including corticosteroids, IVIG, and anti-D.3,7-9 In contrast to some of these approaches, treatment was administered orally in an outpatient setting. It is important to mention that we elected to start eltrombopag after dexamethasone was completed to distinguish immediate responses to and adverse events of each drug. The results achieved were poorer than those obtained by using dexamethasone/rituximab (6-month RFS 75% vs 84%, respectively),9 but better than those obtained in a recent prospective study, in which only 37% of patients in the dexamethasone arm had >50 × 109/L at 6 months vs 75% in our study.10 Our approach could be superior to corticosteroid treatment because sustained responses seem to be higher with this combination.
Impaired megakaryopoiesis and autoreactive T-regulator cells (Tregs) have increasingly been recognized as physiopathological mechanisms involved in ITP.16 In this setting, eltrombopag increases Treg activity and therefore may play a role in altering the natural history of the disease, making the use of this drug in newly diagnosed ITP patients a compelling approach.17 In spite of a limited follow-up, the median DOR (8.3 months) suggests that lasting remissions can be obtained without immunosuppression by adding a short eltrombopag course to dexamethasone. Interestingly, a recent study reported durable remissions in 9 patients with pretreated chronic ITP even after suspending the use of TPO receptor agonists, including eltrombopag.18
Treatment cost is another issue to consider. The prices of ITP drugs (United States) for 1 month, or a single course of treatment in an adult, are approximately prednisone $16, eltrombopag $5934, IVIG (80 g) $9648, and rituximab (2 g) $15 596.19 This is relevant because approximately 25% of adults receive IVIG as initial therapy20 ; the cost of which is substantially higher than the cost of dexamethasone plus eltrombopag. Because there were patients who did not respond to dexamethasone but did respond to eltrombopag, we suggest that TPO mimetics could be a useful option for patients who do not respond initially to steroids, but not yet as a single first-line therapy. Limitations in our study include a small sample size, short follow-up, and the lack of a comparative randomized design. In summary, we found that this combination can be a feasible frontline therapy in ITP, but obviously further investigation in this setting is needed.
Presented in an abstract form at the 55th meeting of the American Society of Hematology, New Orleans, LA, December 2013.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.G.-A., M.A.H.-R., and J.C.J.-P. designed the research; M.A.H.-R., O.G.C.-R., C.H.G.-A., J.H.-R., and G.J.R.-A. performed research; J.H.-R. collected data; L.T.-A. and A.G.-D.L. analyzed and interpreted data; A.G.-D.L. performed statistical analysis; and D.G.-A., J.C.J.-P., O.G.C.-R., C.H.G.-A., L.T.-A., and G.J.R.-A. wrote the manuscript.
Conflict-of interest-disclosure: The authors declare no competing financial interests.
Correspondence: David Gómez-Almaguer, Hematology Service, Hospital Universitario “Dr José Eleuterio González,” Universidad Autónoma de Nuevo León, Madero y Gonzalitos. Col. Mitras Centro, Monterrey, Nuevo León, México; e-mail: dgomezalmaguer@gmail.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal