In this issue of Blood, Roccaro et al demonstrate high expression of C-X-C chemokine receptor type 4 (CXCR4) mutation in Waldenström macroglobulinemia (WM), a marker of tumor aggression and drug resistance.1 In vivo analysis with an anti-CXCR4 antibody reversed this aggression, setting the stage for prospective studies in this forgotten disease.
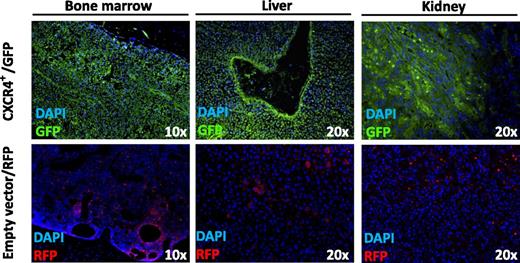
CXCR4+ overexpressing WM cells with aggressive ability to disseminate to bone marrow, liver, and kidney in SCID/Bg mice as demonstrated by immunofluorescence imaging of GFP-tagged CXCR4+ WM cells. DAPI, 4′,6 diamidino-2-phenylindole; RFP, red fluorescent protein. Adapted from Figure 2D in the article by Roccaro et al that begins on page 4120.
CXCR4+ overexpressing WM cells with aggressive ability to disseminate to bone marrow, liver, and kidney in SCID/Bg mice as demonstrated by immunofluorescence imaging of GFP-tagged CXCR4+ WM cells. DAPI, 4′,6 diamidino-2-phenylindole; RFP, red fluorescent protein. Adapted from Figure 2D in the article by Roccaro et al that begins on page 4120.
Lymphoplasmacytic lymphoma, otherwise known as WM, is a rare B-cell malignant disease with an incidence of 3 per million people per year; there are approximately 1400 new diagnoses in the United States per year.2,3 With a median survival of only 5 years from diagnosis, there is no standard therapy or cure for WM and no agents have been specifically approved by the US Food and Drug Administration. In part, this is due to the rarity of disease-limiting preclinical studies to determine high-risk features that predict poor prognosis and, therefore, any clinical studies with targeted therapies to these high-risk prognostic markers.
The study by Roccaro et al, I hope, is about to change this with their findings of the clinical relevance of the C1013G/CXCR4 mutation in patients with WM.1 In a series of elegant studies, Roccaro et al first determined that CXCR4, one of the main regulators of tumor B-cell homing to bone marrow is mutated in almost 30% of patients with WM and 20% in patients with immunoglobulin M (IgM) monoclonal gammopathy of undetermined significance, with very little or no mutation in the other B-cell disorders and healthy volunteers. CXCR4 mutation may be more prevalent in patients with extramedullary disease, as demonstrated by increased CXCR4 mutation in these patients. Roccaro et al next showed a more aggressive dissemination to bone marrow and extramedullary sites when green fluorescence protein (GFP)-tagged CXCR4+ WM cells were injected into severe combined immunodeficiency (SCID)/Bg mice (see figure), which correlated with an increase in serum IgM secretion and an overall reduced survival as compared with empty-vector WM cells. Finally, what makes this study more compelling is the finding that CXCR4-expressing WM cells harbor high resistance to Bruton tyrosine kinase–, phosphatidylinositol 3-kinase–, and mammalian target of rapamycin–targeted drugs ibrutinib, idelalisib, and everolimus, respectively, but excellent response demonstrated in vitro with proteasome inhibitors bortezomib and carfilzomib and both in vivo and in vitro with anti-CXCR4 monoclonal antibody BMS-936564/MDX-1338.
The importance of these findings cannot be overstated. Although other studies have identified CXCR4 as a prominent mutation in WM and shown clinical correlation with poor survival,4,5 this is the first article demonstrating the clinical impact of the mutation with resistance to certain targeted therapies but excellent response to proteasome inhibitors and anti-CXCR4 monoclonal antibody. This holds great promise in patients with a high burden of disease with extramedullary involvement that, according to this study, may have higher overexpression of CXCR4 mutation and who do not respond or have very short response to currently available treatments. A prognostic model that includes CXCR4+ mutation in addition to the current International Prognostic Scoring System for WM (age, hemoglobin, platelet, β-2 microglobulin, and serum monoclonal protein)6 is in the foreseeable future. The percentage of this mutation is high (30%), and the ease of testing for CXCR4 mutation in any tissue including bone marrow calls for urgent prospective clinical phase 1 and 2 studies evaluating anti–CXCR4-targeted therapies as a single agent and in combination with proteasome inhibitors.
This study should set the trend for continued research in this forgotten disease to determine markers of good and poor prognosis that will help guide treatment algorithm.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal