Key Points
ADAM10 but not ADAM17 on leukocytes is essential for chemokine-induced signaling, adhesion, cytoskeletal rearrangement, and migration.
Leukocyte-expressed ADAM10 promotes leukocyte recruitment and edema formation in a murine model of acute pulmonary inflammation.
Abstract
Inflammation is a key process in various diseases, characterized by leukocyte recruitment to the inflammatory site. This study investigates the role of a disintegrin and a metalloproteinase (ADAM) 10 and ADAM17 for leukocyte migration in vitro and in a murine model of acute pulmonary inflammation. Inhibition experiments or RNA knockdown indicated that monocytic THP-1 cells and primary human neutrophils require ADAM10 but not ADAM17 for efficient chemokine-induced cell migration. Signaling and adhesion events that are linked to cell migration such as p38 and ρ GTPase-family activation, F-actin polymerization, adhesion to fibronectin, and up-regulation of α5 integrin were also dependent on ADAM10 but not ADAM17. This was confirmed with leukocytes isolated from mice lacking either ADAM10 or ADAM17 in all hematopoietic cells (vav 1 guanine nucleotide exchange factor [Vav]-Adam10−/− or Vav-Adam17−/− mice). In lipopolysaccharide-induced acute pulmonary inflammation, alveolar recruitment of neutrophils and monocytes was transiently increased in Vav-Adam17−/− but steadily reduced in Vav-Adam10−/− mice. This deficit in alveolar leukocyte recruitment was also observed in LysM-Adam10−/− mice lacking ADAM10 in myeloid cells and correlated with protection against edema formation. Thus, with regard to leukocyte migration, leukocyte-expressed ADAM10 but not ADAM17 displays proinflammatory activities and may therefore serve as a target to limit inflammatory cell recruitment.
Introduction
Leukocyte recruitment and tissue infiltration are early key events in acute inflammation. The recruitment is a multistep process involving rolling, capture, firm adhesion, transendothelial migration, and chemotaxis toward the inflammatory site. This sequence of steps is regulated by an interplay of several soluble and surface expressed mediators.1 Adhesion molecules promote the adhesive interaction of leukocytes and endothelial cells. Cytokine-induced chemokine production is critical for mediating arrest of the leukocytes and directional migration, leading to transmigration of the endothelial cell layer. Subsequently, leukocytes cross the basement membrane and follow a chemotactic gradient toward the site of inflammation. Prototype chemokines are CC-chemokine ligand 2 (CCL2/monocyte chemoattractant protein-1) that recruits monocytic cells and CXC chemokine ligand 8 (CXCL8/interleukin-8 [IL-8]) or murine CXCL1/KC that attracts neutrophils.1
Cell recruitment by classical cytokines, chemokines, and adhesion molecules is further regulated by several other molecules present on the cell surface, including members of the a disintegrin and metalloproteinase (ADAM) family.2 Although protease-independent activities of ADAMs are often carried out by their disintegrin domain and contribute to adhesive cell interactions, the proteolytic activity is mediated by the metalloproteinase domain and mediates the shedding of other surface molecules from the cell membrane. ADAM10 and ADAM17 have especially been related to shedding of inflammatory substrate molecules by leukocytes or endothelial cells.3-5 Some substrates are shed by ADAM17 (eg, tumor necrosis factor [TNF] and l-selectin), others by ADAM10 (eg, most cadherins), and some by both proteases (eg, IL-6 receptor [IL6R] and CX3C-chemokine ligand 1 [CX3CL1]). The fact that ADAM10- or ADAM17-deficient mice die early in development or shortly after birth, respectively,6,7 necessitates studying conditional and/or cell-specific knockout of the proteases. On leukocytes, TNF is predominantly shed by ADAM17, and this cleavage is critically involved in lipopolysaccharide (LPS)-induced septic shock.8 Also l-selectin on neutrophils is shed by ADAM17, which down-regulates neutrophil adhesiveness and recruitment to inflammatory sites.9,10 At present, no information exists on leukocyte-specific knockout of ADAM10 in models of acute pulmonary inflammation.
This study compares the contribution of leukocyte-expressed ADAM10 and ADAM17 to the migration and inflammatory recruitment of leukocytes in vitro and in acute pulmonary inflammation in vivo. Pharmacological inhibition and transcriptional silencing demonstrate that ADAM10 but not ADAM17 is essential for chemokine-induced migration of human and murine monocytic cells and neutrophils. This associates with the involvement of ADAM10 in chemokine-induced signaling, adhesion, cytoskeletal rearrangement, and integrin upregulation. Mice with selective protease deficiency in hematopoietic or myeloid cells were analyzed in a model of LPS-induced acute pulmonary inflammation. The results indicate that ADAM10 but not ADAM17 on hematopoietic cells promotes alveolar recruitment of tissue infiltrating leukocytes and development of pulmonary edema.
Materials and methods
Antibodies, chemokines, and inhibitors
LPS was from Escherichia coli strain 0127:B8 (Sigma-Aldrich, Munich, Germany); other sources are listed in the supplemental Materials available on the Blood Web site.
Mice
Vav 1 guanine nucleotide exchange factor (Vav)-Adam10−/− mice and Vav-Adam17−/− mice expressed Cre recombinase under control of the Vav promotor and were homozygous for floxed Adam10 or floxed Adam17, respectively.8,11 Lysozyme M (LysM)-Adam10−/− mice expressed Cre recombinase via the LysM promotor and were homozygous for floxed Adam10. Littermates (litter control) of the same background expressed Cre recombinase but no floxed protease genes (supplemental Materials). Animal experiments were approved by the local authorities and performed with 6- to 8-week-old female mice on a C57BL/6 background (82-02.04.2011.A335 and 87-51.04.2010.A026; LANUV NRW).
Cell culture and cell preparation
THP-1 cells, lung human microvascular endothelial cells (HMVEC-L), human embryonic kidney cells (HEK293), and human epithelial bladder carcinoma cells (ECV304) were cultured as previously described.12-14 For isolation and culture of human and murine blood cells, see the supplemental Materials.
Lentiviral transduction
Short hairpin RNA (shRNA) targeting ADAM10 and ADAM17 was inserted into the lentiviral expression vector pLVTHM (Addgene plasmid 12247), and lentiviral particles were prepared and used for transduction as previously described.15 The shRNA sequences are detailed in the supplemental Materials.
Quantitative reverse transcriptase-polymerase chain reaction
mRNA levels for the indicated genes in murine bone marrow-derived macrophages (BMDMs) and human THP-1 cells were quantified by quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) analysis and normalized to the mRNA level of murine RPS29 or human glyceraldehyde-3-phosphate dehydrogenase as previously described.12 For primer sequences, see the supplemental Materials.
Flow cytometric analysis
Cells were analyzed for surface expression of ADAM10 and ADAM17 as previously described.15 The same procedure was used to study integrin expression with antibodies against either total or active integrin subunits. For analysis of murine bronchalveolar lavage (BAL) fluid, blood samples, neutrophil, macrophage, and lymphocyte gates were defined by staining for CD11b, CD4, CD8e, Ly6G, and F4/80 and cross-checked with the appropriate isotype controls (supplemental Materials).12
Chemotaxis, transmigration, and adhesion assay
Phalloidin stain
F-actin polymerization in THP-1 cells and BMDMs was measured by phalloidin stain and is detailed in the supplemental Materials.
Extracellular signal-regulated kinase 1/2, p38 activation, and Rho GTPase activation
Assays were performed with commercial antibodies and kits according to the manufacturer. See the supplemental Materials for details.
LPS-induced acute pulmonary inflammation
The LPS-induced model of acute pulmonary inflammation has been described and is detailed in the supplemental Materials.12
Statistics
Quantitative data are shown as mean ± standard error of the mean (SEM) calculated from ≥3 independent experiments and cell isolates. Animal numbers per group are indicated within the figure legends. Percentage data were arc sin-transformed. Statistics were calculated using PRISM5.0 (GraphPad Software, La Jollla, CA). Where applicable, data were analyzed by 1-way analysis of variance (ANOVA) followed by Bonferroni correction, and differences were indicated by crosses (+++P < .0001, ++P < .01, +P < .05). To test for a difference to a single hypothetical value (eg, 100%), the 1-sample t test was used, and differences were indicated by asterisks (***P < .0001, **P < .01, *P < .05).
This study was conducted in accordance with the Declaration of Helsinki.
Results
ADAM10 is required for monocytic cell migration
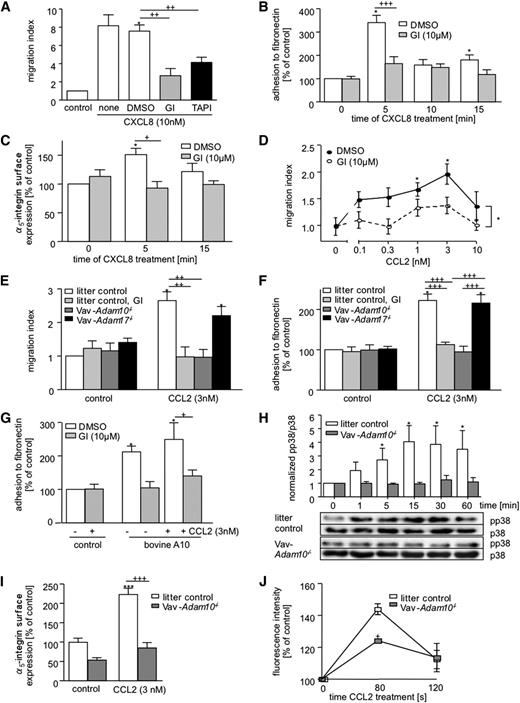
The contribution of ADAM10 and ADAM17 to leukocyte migration was studied by chemotaxis experiments with THP-1 cells using the CC chemokine CCL2 as a chemoattractant. Pretreatment of cells with the metalloproteinase inhibitor GI254023X blocking ADAM10 with 100-fold more potency than ADAM1717 reduced CCL2-induced chemotaxis in a concentration-dependent manner, reaching half-maximal inhibition at approximately 1 µM (Figure 1A), corresponding to the reported IC50 value for inhibition of cell-expressed ADAM10.18,19 The inhibitor TAPI-1 predominantly blocks ADAM17,15 but also inhibits ADAM10 at higher concentrations. This inhibitor had a less pronounced effect on cell migration (Figure 1B). There also was an effect of GI254023X on random migration in the absence of chemotactic stimulus, but compared with the inhibition of chemokine-induced migration, the effect was rather small (supplemental Figure 1A). When migration experiments were extended to longer time periods, the migratory response to CCL2 reached a maximum at 4 hours, whereas cells treated with GI254023X showed a delayed response still increasing over a period up to 6 hours (supplemental Figure 1B).
Effect of metalloproteinase inhibitors and ADAM10/ADAM17 knockdown on THP-1 cell migration. (A-B) THP-1 cells were preincubated with varying concentrations of the inhibitors (A) GI254023X (GI) in or (B) TAPI-1 or appropriate dilutions of vehicle (dimethylsulfoxide [DMSO]) for 15 minutes and then assayed for cell migration in response to 3 nM CCL2. The number of migrated cells in the lower compartment was determined by measurement of endogenous glucuronidase activity, and the results are expressed in relation to cells receiving no chemoattractant and no inhibitor. Migration of cells receiving no chemoattractant is indicated by dotted lines. (C) THP-1 cells were pretreated with GI254023X (10 µM) or DMSO (0.1%) for 30 minutes. Subsequently, cells were either directly assayed for CCL2-induced cell migration or washed to remove free inhibitor. Washed cells were either directly investigated for CCL2-induced chemotaxis or further incubated for 60 minutes (recovery) before the assay. Results are expressed in relation to the untreated control receiving no chemoattractant (dotted line). (D) THP-1 cells were transduced with different lentivirus encoding scramble-shRNA (scr), ADAM10-shRNA (A10-650 and A10-1947), or ADAM17-shRNA (A17-2061 and A17-2646) and assayed for CCL2-induced cell migration. Results are expressed in relation to the scramble control receiving no chemoattractant (dotted line). (E) Following CCL2 stimulation in the presence of 10 µM GI254023X or 0.1% DMSO (15-minute pretreatment), cells were removed from the top of the chemotaxis membrane, and the remaining cells were stained in the chemotaxis membrane. (Left) Representative images and (right) quantitative data are shown. (F) Green fluorescent protein-expressing THP-1 cells were pretreated with 10 µM GI254023X or 0.1% DMSO and subsequently investigated for transmigration through dsRed-expressing ECV304 cells grown on transwell filters. THP-1 cells were (left) visualized by confocal microscopy and Z-stack analysis and (right) quantified within (pos.1) and below (pos.2) the ECV304 layer. Quantitative data represent means ± SEM of 3 independent experiments. Significance was calculated using 1-way ANOVA and the Bonferroni post-test and is indicated by crosses. Asterisks without a line indicate significant differences to the nontreated control analyzed by 1-sample t test.
Effect of metalloproteinase inhibitors and ADAM10/ADAM17 knockdown on THP-1 cell migration. (A-B) THP-1 cells were preincubated with varying concentrations of the inhibitors (A) GI254023X (GI) in or (B) TAPI-1 or appropriate dilutions of vehicle (dimethylsulfoxide [DMSO]) for 15 minutes and then assayed for cell migration in response to 3 nM CCL2. The number of migrated cells in the lower compartment was determined by measurement of endogenous glucuronidase activity, and the results are expressed in relation to cells receiving no chemoattractant and no inhibitor. Migration of cells receiving no chemoattractant is indicated by dotted lines. (C) THP-1 cells were pretreated with GI254023X (10 µM) or DMSO (0.1%) for 30 minutes. Subsequently, cells were either directly assayed for CCL2-induced cell migration or washed to remove free inhibitor. Washed cells were either directly investigated for CCL2-induced chemotaxis or further incubated for 60 minutes (recovery) before the assay. Results are expressed in relation to the untreated control receiving no chemoattractant (dotted line). (D) THP-1 cells were transduced with different lentivirus encoding scramble-shRNA (scr), ADAM10-shRNA (A10-650 and A10-1947), or ADAM17-shRNA (A17-2061 and A17-2646) and assayed for CCL2-induced cell migration. Results are expressed in relation to the scramble control receiving no chemoattractant (dotted line). (E) Following CCL2 stimulation in the presence of 10 µM GI254023X or 0.1% DMSO (15-minute pretreatment), cells were removed from the top of the chemotaxis membrane, and the remaining cells were stained in the chemotaxis membrane. (Left) Representative images and (right) quantitative data are shown. (F) Green fluorescent protein-expressing THP-1 cells were pretreated with 10 µM GI254023X or 0.1% DMSO and subsequently investigated for transmigration through dsRed-expressing ECV304 cells grown on transwell filters. THP-1 cells were (left) visualized by confocal microscopy and Z-stack analysis and (right) quantified within (pos.1) and below (pos.2) the ECV304 layer. Quantitative data represent means ± SEM of 3 independent experiments. Significance was calculated using 1-way ANOVA and the Bonferroni post-test and is indicated by crosses. Asterisks without a line indicate significant differences to the nontreated control analyzed by 1-sample t test.
Effective inhibition by GI254023X was achieved by a short preincubation time (15-30 minutes). When cells were washed directly before the migration assay to remove free inhibitor, cell migration was still suppressed (Figure 1C). However, when inhibitor-treated cells were further incubated for 60 minutes in the absence of inhibitor and then assayed for chemotaxis, the response recovered. This excludes the possibility that the effect of GI254023X is mediated by induction of long-term adaptations or by general effects on cell viability.
The inhibition experiments suggested that ADAM10 could be critically involved in cell migration but did not rule out an additional contribution of ADAM17. Therefore, ADAM10 and ADAM17 in THP-1 cells were downregulated on the transcriptional level using lentiviral vectors coding for specific shRNA (2 different shRNA vectors for each ADAM protease or a scramble control shRNA vector). Effective downregulation of ADAM10 and ADAM17 expression was shown by qRT-PCR and flow cytometry (supplemental Figure 1C-D). The knockdown of ADAM10 reduced migration in response to CCL2 (Figure 1D), whereas the knockdown of ADAM17 had no effect. To clarify whether a similar effect could be seen for transendothelial migration of THP-1 cells, human microvascular endothelial cells of the lung (HMVEC-L) were cultured on transwell filters. CCL2-induced transendothelial migration of THP-1 cells was also suppressed by ADAM10 silencing but not by ADAM17 silencing (supplemental Figure 1E).
Next, we investigated which step of migration is affected by the ADAM10 inhibitor GI254023X. CCL2 increased migration through the pores of the chemotaxis membranes, and cells that were still in the process of transmigrating through the pores could be visualized by staining (Figure 1E). Of note, GI254023X increased the number of cells that remained within the pores. Again, a very similar observation was made on silencing of ADAM10, whereas silencing of ADAM17 had no effect (supplemental Figure 1F). Thus, leukocytes require ADAM10 to effectively migrate through the pores and finally detach from the filter. This was further analyzed in a transcellular migration setup in which THP-1 cells transmigrated through a layer of ECV-304 cells. These cells do not represent endothelial cells, but they can be grown as a monolayer that is thick enough for confocal Z-stack analysis to visualize migrating leukocytes on, within, or below the ECV304 cell layer. In the presence of GI254023X, THP-1 cells were able to migrate into the ECV304 cell layer (pos.1) but were unable to migrate further to the lower side of the membrane (pos.2; Figure 1F).
ADAM10 promotes chemokine-induced signaling, adhesion to fibronectin, and α5 integrin surface expression
We next investigated possible effects of ADAM10 on CCL2-induced intracellular signals involved in the regulation of adhesion and chemotaxis. Assessment of intracellular calcium transients in response to CCL2 did not indicate any influence of GI254023X or knockdown of ADAM10 or 17 (supplemental Figure 2A-D). We then studied the influence of CCL2-induced phosphorylation of extracellular signal-regulated kinase (ERK)1/2 and p38 kinase. Inhibition by U0126 and SB203580 confirmed that ERK1/2 and p38 signaling is important for CCL2-mediated THP-1 cell migration (supplemental Figure 2E). In the presence of GI254023X, phosphorylation of ERK1/2 in response to CCL2 was slightly reduced at early time points of stimulation (5 minutes), but this difference vanished at later time points (Figure 2A). Phosphorylation of p38, however, was more efficiently suppressed over a longer time period. This was confirmed by shRNA-mediated silencing of ADAM10, leading to inhibition of early ERK1/2 phosphorylation and p38 phosphorylation in response to CCL2 (Figure 2B). Because ERK and p38 activation might arise from stimulation by the epidermal growth factor receptor (EGFR) that can be activated by metalloproteinase-dependent shedding of growth factors,20 we next probed the EGFR inhibitors PD168393 and cetuximab for their effect on CCL2-induced chemotaxis. In fact, cell migration was reduced by both inhibitors (supplemental Figure 2F).
Effect of ADAM10 inhibition or knockdown on CCL2-induced signaling, adhesion, and F-actin polymerization in THP-1 cells. (A-B) THP-1 cells were (A) treated with 10 µM GI254023X or DMSO for 15 minutes or (B) transduced with lentivirus encoding shRNA targeting ADAM10 or scramble shRNA. Subsequently, cells were assayed for CCL2-induced phosphorylation of ERK1/2 and p38 at the indicated time points by western blotting. Samples treated with DMSO and GI254023X were run on the same gel. Total ERK1/2 and p38 levels were determined in parallel (separate blots had to be used for pp38). Signals were quantified by densitometry, normalized to the expression of total kinase, and expressed in relation to the phosphorylation level of (A) the unstimulated control (0 minutes) for DMSO-treated THP-1 cells and (B) separately for each transduced THP-1 cell type. (C-F) THP-1 cells were pretreated with 10 µM GI254023X or 0.1% DMSO for 15 minutes and stimulated with CCL2 (3 nM) or left unstimulated. Subsequently, cells were assayed for (C) binding to coated fibronectin, (D-E) upregulation of α5 integrin surface expression (D, representative histograms; E, quantification), and (F) Rho GTPase activation. Rho activation was quantified as active Rho protein in relation to total Rho protein as determined by western blotting. (G) THP-1 cells were treated with 10 µM GI254023X or transduced with lentivirus to downregulate ADAM10. DMSO and scramble shRNA were used as controls. Subsequently, cells were assayed for polymerization of F-actin by flow cytometry using fluorophore-labeled phalloidin. Results were expressed as percentage of the controls (DMSO or scramble). Quantitative data represent means ± SEM of 3 independent experiments. Crosses indicate significance among treated cells calculated using 1-way ANOVA and the Bonferroni post-test. Asterisks without a line indicate significant differences to the nontreated control analyzed by 1-sample t test.
Effect of ADAM10 inhibition or knockdown on CCL2-induced signaling, adhesion, and F-actin polymerization in THP-1 cells. (A-B) THP-1 cells were (A) treated with 10 µM GI254023X or DMSO for 15 minutes or (B) transduced with lentivirus encoding shRNA targeting ADAM10 or scramble shRNA. Subsequently, cells were assayed for CCL2-induced phosphorylation of ERK1/2 and p38 at the indicated time points by western blotting. Samples treated with DMSO and GI254023X were run on the same gel. Total ERK1/2 and p38 levels were determined in parallel (separate blots had to be used for pp38). Signals were quantified by densitometry, normalized to the expression of total kinase, and expressed in relation to the phosphorylation level of (A) the unstimulated control (0 minutes) for DMSO-treated THP-1 cells and (B) separately for each transduced THP-1 cell type. (C-F) THP-1 cells were pretreated with 10 µM GI254023X or 0.1% DMSO for 15 minutes and stimulated with CCL2 (3 nM) or left unstimulated. Subsequently, cells were assayed for (C) binding to coated fibronectin, (D-E) upregulation of α5 integrin surface expression (D, representative histograms; E, quantification), and (F) Rho GTPase activation. Rho activation was quantified as active Rho protein in relation to total Rho protein as determined by western blotting. (G) THP-1 cells were treated with 10 µM GI254023X or transduced with lentivirus to downregulate ADAM10. DMSO and scramble shRNA were used as controls. Subsequently, cells were assayed for polymerization of F-actin by flow cytometry using fluorophore-labeled phalloidin. Results were expressed as percentage of the controls (DMSO or scramble). Quantitative data represent means ± SEM of 3 independent experiments. Crosses indicate significance among treated cells calculated using 1-way ANOVA and the Bonferroni post-test. Asterisks without a line indicate significant differences to the nontreated control analyzed by 1-sample t test.
Next, we studied whether ADAM10, ERK1/2, p38, or EGFR inhibition would affect THP-1 cell adhesion to coated integrin ligands, eg, fibronectin or intercellular adhesion molecule (ICAM)-1. Stimulation with CCL2 enhanced binding to fibronectin, and this interaction was reduced by ERK1/2 or p38 inhibition (supplemental Figure 2G). By contrast, EGFR inhibition had no effect on CCL2-induced adhesion to fibronectin, suggesting that EGFR is not relevant for this process (supplemental Figure 2H). Nevertheless, GI254023X completely suppressed adhesion to fibronectin (Figure 2C), indicating that ADAM10 is a key molecule for this type of cell adhesion. The effect of the ADAM10 inhibitor was less pronounced for ICAM-1–mediated adhesion and did not reach significance (supplemental Figure 2I).
Our findings suggested that fibronectin binding by α5β1 integrin and ICAM-1 binding by αMβ2 integrin, which are both expressed by THP-1 cells, could be differentially regulated by ADAM10. Analysis of integrin surface expression by flow cytometry indicated up-regulation of β2, αL, and αM integrins by CCL2, which was not sensitive to treatment with GI254023X (supplemental Figure 2J-L). There was no effect on the surface level of activated β1 integrin (supplemental Figure 2M). By contrast, CCL2-induced upregulation of α5 integrin was suppressed by the ADAM10 inhibitor (Figure 2D-E). Similar to the adhesion experiments, upregulation of α5 integrin was also blocked by inhibition of ERK1/2 and p38 but not by EGFR inhibition (supplemental Figure 2N-O). Because integrin-mediated signals in cell migration involve activation of the small GTPase family Rho GTPase,21 we also studied the effect of ADAM10 inhibition on this pathway. Again we observed profound inhibition of CCL2-mediated Rho GTPase family activation by GI254023X (Figure 2F). Rho GTPase activation is a critical event in chemokine-induced F-actin polymerization, and indeed, treatment with GI254023X or ADAM10 silencing but not ADAM17 silencing abrogated F-actin polymerization in response to CCL2 (Figure 2G).
ADAM10 is required for migration and adhesion of primary human and murine neutrophils and monocytic cells
To clarify whether the observed inhibition of cell migration would also account for other cell types and for primary cells, human neutrophils were assayed for chemotaxis induced by the human neutrophil attracting chemokine CXCL8/IL-8. As seen for CCL2-induced migration of THP-1 cells, CXCL8-induced neutrophil migration was considerably decreased in the presence of GI254023X (Figure 3A; supplemental Figure 3A), and this was associated with an accumulation of cells within the pores of the membrane (supplemental Figure 3B). Inhibition was also observed with TAPI-1 (Figure 3A; supplemental Figure 3C). Adhesion experiments demonstrated that GI254023X reduces CXCL8-induced neutrophil adhesion to fibronectin (Figure 3B) but not to ICAM-1 (supplemental Figure 3D). As seen for THP-1 cells, the inhibitor prevented chemokine-induced upregulation of α5 integrin (Figure 3C).
ADAM10 promotes migration and fibronectin binding of human neutrophils and murine BMDMs. (A-C) Human neutrophils were pretreated with 10 µM GI254023X or 0.1% DMSO or left untreated for 15 minutes and were subsequently assayed for (A) cell migration, (B) adhesion to fibronectin, and (C) α5 integrin surface expression, induced by CXCL8 (10 nM). (D) Murine BMDMs were generated from wild-type mice treated with GI254023X (10 µM) or DMSO (0.1%) and investigated for chemotaxis induced by various concentrations of CCL2. (E-F) Murine BMDM from Vav-Adam10−/− and Vav-Adam17−/− mice and litter controls were pretreated with 10 µM GI254023X or vehicle (DMSO) and investigated for (E) CCL2-induced cell migration and (F) adhesion to fibronectin. (G) ADAM10-deficient BMDMs were transfected with bovine ADAM10, treated with 10 µM GI254023X or 0.1% DMSO, and investigated for CCL2-induced adhesion to fibronectin after 15 minutes. (H) BMDMs from Vav-Adam10−/− and litter control mice were stimulated with murine CCL2 (3 nM) for the indicated time periods and investigated for phosphorylation of p38 by western blotting. Samples were run on the same gel. (I) BMDMs from Vav-Adam10−/− and litter controls were treated for 15 minutes with CCL2 and investigated for upregulation of α5 integrin surface expression. (J) BMDM from Vav-Adam10−/− and litter controls were treated with 3 nM CCL2 for the indicated time periods and examined for polymerization of F-actin by flow cytometry. Quantitative data represent means ± SEM of 3 independent experiments and cell preparations. Crosses indicate significance among treated cells calculated using 1-way ANOVA and the Bonferroni post-test. Asterisks without a line indicate significant differences to the nontreated control analyzed by 1-sample t test.
ADAM10 promotes migration and fibronectin binding of human neutrophils and murine BMDMs. (A-C) Human neutrophils were pretreated with 10 µM GI254023X or 0.1% DMSO or left untreated for 15 minutes and were subsequently assayed for (A) cell migration, (B) adhesion to fibronectin, and (C) α5 integrin surface expression, induced by CXCL8 (10 nM). (D) Murine BMDMs were generated from wild-type mice treated with GI254023X (10 µM) or DMSO (0.1%) and investigated for chemotaxis induced by various concentrations of CCL2. (E-F) Murine BMDM from Vav-Adam10−/− and Vav-Adam17−/− mice and litter controls were pretreated with 10 µM GI254023X or vehicle (DMSO) and investigated for (E) CCL2-induced cell migration and (F) adhesion to fibronectin. (G) ADAM10-deficient BMDMs were transfected with bovine ADAM10, treated with 10 µM GI254023X or 0.1% DMSO, and investigated for CCL2-induced adhesion to fibronectin after 15 minutes. (H) BMDMs from Vav-Adam10−/− and litter control mice were stimulated with murine CCL2 (3 nM) for the indicated time periods and investigated for phosphorylation of p38 by western blotting. Samples were run on the same gel. (I) BMDMs from Vav-Adam10−/− and litter controls were treated for 15 minutes with CCL2 and investigated for upregulation of α5 integrin surface expression. (J) BMDM from Vav-Adam10−/− and litter controls were treated with 3 nM CCL2 for the indicated time periods and examined for polymerization of F-actin by flow cytometry. Quantitative data represent means ± SEM of 3 independent experiments and cell preparations. Crosses indicate significance among treated cells calculated using 1-way ANOVA and the Bonferroni post-test. Asterisks without a line indicate significant differences to the nontreated control analyzed by 1-sample t test.
We further analyzed BMDMs for migration in response to murine CCL2. Treatment with GI254023X again suppressed the migratory response to CCL2 and showed almost no effect on random BMDM migration in the absence of CCL2 (Figure 3D). For the further study of leukocyte-expressed ADAM10 or ADAM17 in mice, animals with the floxed Adam10 or Adam17 gene8,11 were crossed with Vav-cre mice for conditional knockout in hematopoietic cells. BMDMs were generated from these mice and investigated by flow cytometry for F4/80 expression to confirm purity (>95%; supplemental Figure 3E). The selective knockout was demonstrated by flow cytometry for ADAM10 and qRT-PCR for ADAM10 and ADAM17 (supplemental Figure 3F-G).
ADAM10 deficiency in BMDMs reduced CCL2-induced cell migration to a similar extent as the ADAM10 inhibitor (Figure 3E). By contrast, ADAM17 deficiency in these cells did not affect cell migration. As seen for THP-1 cells and neutrophils, GI254023X reduced chemokine-induced BMDM adhesion to fibronectin (Figure 3F) but not to ICAM-1 (supplemental Figure 3H). Moreover, ADAM10 deficiency reduced adhesion to fibronectin to a similar extent as the ADAM10 inhibitor, whereas ADAM17 deficiency had no effect (Figure 3F). Adhesion to ICAM-1 was again not affected by ADAM10 or ADAM17 deficiency (supplemental Figure 3H). Overexpression of ADAM10 in ADAM10-deficient BMDMs reconstituted cell adhesion to fibronectin, which was prevented by GI254023X (Figure 3G). Finally, as seen for THP-1 cells, ADAM10 deficiency in BMDM also prevented chemokine-induced activation of p38 (Figure 3H), upregulation of α5 integrin surface expression (Figure 3I), F-actin polymerization (Figure 3J), and Rho GTPase activation (supplemental Figure 3I)
Subsequently, migration of murine blood leukocytes in response to non–heat-inactivated fetal bovine serum as a chemotactic stimulus was analyzed. As detected by flow cytometry analysis and gating of the migrated cells (supplemental Figure 3K), GI254023X or ADAM10 deficiency but not ADAM17 deficiency reduced the CCL2-induced migration but not the random migration of blood neutrophils (supplemental Figure 3J).
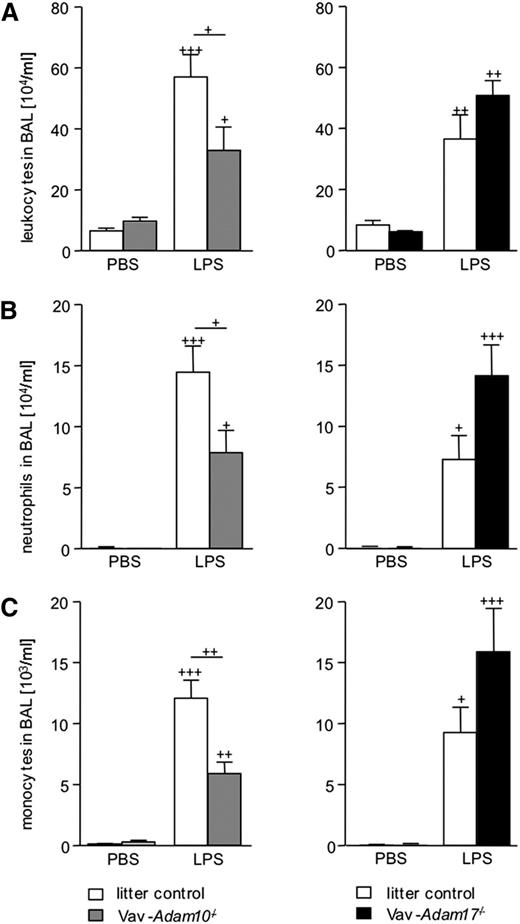
Leukocyte expressed ADAM10 and ADAM17 differentially contribute to alveolar leukocyte recruitment in LPS-induced acute pulmonary inflammation
To investigate the in vivo role of ADAM10 or ADAM17 for leukocyte migration, a model of acute pulmonary inflammation was used.12 LPS was applied intranasally, leading to increased leukocyte infiltration into the lung after 24 hours, which was quantified by flow cytometric analysis of the BAL fluid cells (Figure 4A). In line with previous studies, BAL fluid from LPS-treated litter controls contained more neutrophils and monocytes than phosphate-buffered saline (PBS)-treated control mice (Figure 4B-C).12 At the same time, monocyte and granulocyte counts but not lymphocyte counts in the blood increased as a consequence of LPS treatment (supplemental Figure 4A-B). Compared with the litter controls, neither Vav-Adam10−/− nor Vav-Adam17−/− mice showed any change in blood leukocyte composition within each treatment group (PBS or LPS). Basal leukocyte numbers in the BAL fluid of PBS-treated animals was low and did not differ among the genotypes. However, in LPS-treated animals, the influx of neutrophil and monocyte recruitment into the airspaces was reduced in Vav-Adam10−/− mice compared with the litter controls (Figure 4B-C). By contrast, LPS-treated Vav-Adam17−/− mice showed a tendency to enhanced leukocyte recruitment, although this did not quite reach significance (P = .06 using the Student t test).
Deficiency of ADAM10 abrogates alveolar leukocyte recruitment in LPS-induced acute pulmonary inflammation. (A-C) Vav-Adam10−/−, Vav-Adam17−/− mice, and respective litter controls were intranasally treated with 400 µg/kg LPS or vehicle (PBS). After 24 hours, lungs were lavaged, and BAL fluid was investigated for (A) content of leukocytes, (B) neutrophils, and (C) monocytes by flow cytometry. Results are expressed as cell number per milliliter of BAL fluid. Data represent means ± SEM (n = 5). Significance was calculated using 1-way ANOVA and the Bonferroni post-test and is indicated by crosses. Crosses without a line indicate significance to the PBS-treated control.
Deficiency of ADAM10 abrogates alveolar leukocyte recruitment in LPS-induced acute pulmonary inflammation. (A-C) Vav-Adam10−/−, Vav-Adam17−/− mice, and respective litter controls were intranasally treated with 400 µg/kg LPS or vehicle (PBS). After 24 hours, lungs were lavaged, and BAL fluid was investigated for (A) content of leukocytes, (B) neutrophils, and (C) monocytes by flow cytometry. Results are expressed as cell number per milliliter of BAL fluid. Data represent means ± SEM (n = 5). Significance was calculated using 1-way ANOVA and the Bonferroni post-test and is indicated by crosses. Crosses without a line indicate significance to the PBS-treated control.
ADAM10 or ADAM17 in hematopoietic cells differentially contributes to early leukocyte tissue infiltration, edema formation, and cytokine release
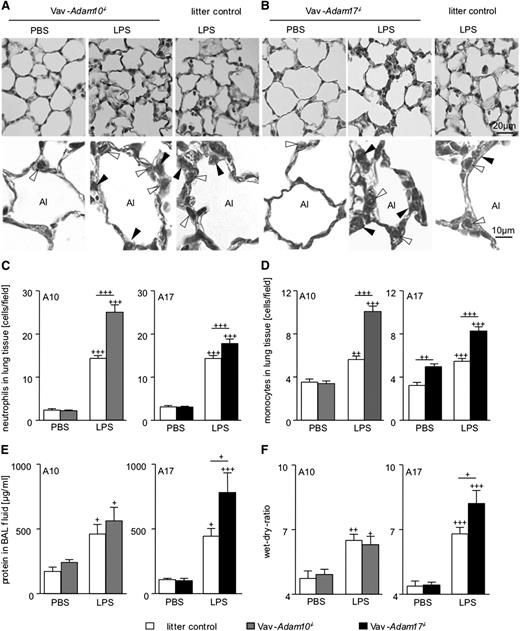
To study whether differences in alveolar leukocyte recruitment would be related to differences in leukocyte tissue infiltration, lungs were perfused to remove vessel-attached leukocytes, histological analysis of lung sections was performed (Figure 5A-B), and tissue resident neutrophils and monocytes were counted. As expected, LPS treatment of Adam10 and Adam17 litter control mice profoundly increased the number of neutrophils and monocytes within the tissue (Figure 5C-D). Of note, LPS-treated Vav-Adam10−/− mice contained even more neutrophils and monocytes in the lung tissue. Taking into account that leukocytes of Vav-Adam10−/− mice did not further migrate into the BAL fluid as shown by reduced BAL cell numbers, this indicates that cells accumulate in the tissue when crossing the endothelium. Lungs of LPS-treated Vav-Adam17−/− mice also contained more leukocytes within the lung tissue than LPS-treated litter control mice.
Influence of ADAM10 or ADAM17 deficiency on cell recruitment to lung tissue and on edema formation. (A-F) Vav-Adam10−/− mice, Vav-Adam17−/− mice, and respective litter controls were intranasally treated with 400 µg/kg LPS or vehicle (PBS). Three-micrometer sections of formalin-fixed and paraffin-embedded lung tissue were stained with hematoxylin-eosin to differentiate monocytic and polymorphnuclear cells. Representative overview images are shown for (A) Vav-Adam10−/− mice and (B) Vav-Adam17−/− mice (upper). Alveolar lumen (Al), neutrophils (filled arrowheads), and monocytic cells (open arrowheads) were marked in the high magnifications (lower). Ten images per animal were analyzed for the recruitment of (C) neutrophils and (D) monocytes using AixoVision software. Twenty-four hours after application, (E) alveolar protein influx was determined and (F) lung wet-dry-ratio was determined. Quantitative data represent means ± SEM (n = 5). Significance was calculated using 1-way ANOVA and the Bonferroni post-test and is indicated by crosses. Crosses without a line indicate significance to the PBS-treated control. Bar legends are the same for C and E and D and F.
Influence of ADAM10 or ADAM17 deficiency on cell recruitment to lung tissue and on edema formation. (A-F) Vav-Adam10−/− mice, Vav-Adam17−/− mice, and respective litter controls were intranasally treated with 400 µg/kg LPS or vehicle (PBS). Three-micrometer sections of formalin-fixed and paraffin-embedded lung tissue were stained with hematoxylin-eosin to differentiate monocytic and polymorphnuclear cells. Representative overview images are shown for (A) Vav-Adam10−/− mice and (B) Vav-Adam17−/− mice (upper). Alveolar lumen (Al), neutrophils (filled arrowheads), and monocytic cells (open arrowheads) were marked in the high magnifications (lower). Ten images per animal were analyzed for the recruitment of (C) neutrophils and (D) monocytes using AixoVision software. Twenty-four hours after application, (E) alveolar protein influx was determined and (F) lung wet-dry-ratio was determined. Quantitative data represent means ± SEM (n = 5). Significance was calculated using 1-way ANOVA and the Bonferroni post-test and is indicated by crosses. Crosses without a line indicate significance to the PBS-treated control. Bar legends are the same for C and E and D and F.
To assess edema formation, the alveolar influx of protein in response to LPS and the lung wet-to-dry ratio were measured (Figure 5E-F). After 24 hours of LPS exposure, this response was, however, not affected by the Vav-Adam10−/− genotype. Thus, leukocyte arrest in the tissue and decreased alveolar leukocyte recruitment were not associated with a reduction in tissue damage. By contrast, in Vav-Adam17−/− mice, increased edema formation and protein influx were observed, correlating with the increased tissue infiltration and alveolar recruitment of leukocytes in these mice.
Vav-Adam10−/− and Vav-Adam17−/− mice were also analyzed for protein levels of proinflammatory mediators in the BAL fluid. As expected, LPS stimulation increased the release of TNF, CCL2, CXCL1, and sIL6R in the BAL fluid of the litter controls (supplemental Figure 5A-D). ADAM10 deficiency reduced LPS-induced CXCL1 and IL6R release, whereas ADAM17 deficiency showed a tendency to enhance CCL2 release, although this did not reach significance (P = .06). Of note, neither ADAM10 nor ADAM17 deficiency in leukocytes affected TNF release into the BAL fluid, suggesting that cell types other than leukocytes are more relevant for the release of soluble TNF into the alveolar lumen.
Deficiency of ADAM10 reduces edema formation at a later stage
To investigate a potential long-term effect of leukocyte-expressed ADAM10 and ADAM17 on lung tissue inflammation, we extended the time of LPS treatment to 72 hours. At this time point, the numbers of neutrophils and monocytes in the blood were not increased in response to LPS treatment. Again, we could not observe a difference in blood leukocyte composition among the genotypes (supplemental Figure 6A-B). At 72 hours after LPS exposure, monocyte and granulocyte recruitment was less compared with the 24-hour time point, but still there was a reduction in Vav-Adam10−/− mice (Figure 6A-B) but not in Vav-Adam17−/− mice (Figure 5D-E) compared with the litter controls. Importantly, at this later stage of the inflammatory response, ADAM10 deficiency was associated with a protection against edema formation (Figure 6C), whereas ADAM17 deficiency had no influence (Figure 6F). To further support the role of ADAM10 in a later stage of pulmonary inflammation, we extended the study to mice with LysM-driven deficiency of ADAM10 in myeloic cells. Deficiency of ADAM10 in these cells was confirmed by analysis of BMDMs for ADAM10 mRNA and protein surface expression (supplemental Figure 6C-D). Compared with litter controls, LysM-Adam10−/− mice did not show a difference in blood leukocyte composition (supplemental Figure 6E). Again, 72 hours after LPS treatment, we observed a reduced neutrophil and monocyte recruitment into the alveolar lumen (Figure 6G-H), which was associated with a protection against edema formation (Figure 6I).
ADAM10 deficiency attenuates LPS-induced acute pulmonary inflammation. (A-C) Vav-Adam10−/−, (D-F) Vav-Adam17−/−, (G-I) LysM-Adam10−/−, and respective litter control mice were intranasally treated with 400 µg/kg LPS or vehicle (PBS). After 72 hours, lungs were lavaged, and BAL fluid was analyzed for content of (A,D,G) neutrophils and (B,E,H) monocytes by flow cytometry. Results are expressed as cell number per milliliter of BAL fluid. (C,F,I) After 72 hours, lung wet-dry-ratio was determined. Data represent means ± SEM (n = 5). Significance was calculated using 1-way ANOVA and the Bonferroni post-test and is indicated by crosses. Crosses without a line indicate significance to the PBS-treated control.
ADAM10 deficiency attenuates LPS-induced acute pulmonary inflammation. (A-C) Vav-Adam10−/−, (D-F) Vav-Adam17−/−, (G-I) LysM-Adam10−/−, and respective litter control mice were intranasally treated with 400 µg/kg LPS or vehicle (PBS). After 72 hours, lungs were lavaged, and BAL fluid was analyzed for content of (A,D,G) neutrophils and (B,E,H) monocytes by flow cytometry. Results are expressed as cell number per milliliter of BAL fluid. (C,F,I) After 72 hours, lung wet-dry-ratio was determined. Data represent means ± SEM (n = 5). Significance was calculated using 1-way ANOVA and the Bonferroni post-test and is indicated by crosses. Crosses without a line indicate significance to the PBS-treated control.
Discussion
The present study provides in vivo and in vitro evidence that leukocytes need ADAM10 but not ADAM17 for their migration. Pharmacological inhibition, gene silencing, and gene knockout experiments consistently demonstrate that ADAM10 is necessary for efficient migration of human and murine monocytic cells and neutrophils in response to the chemokines CCL2 and CXCL8, respectively. Moreover, ADAM10 on leukocytes is vital for p38 signaling, Rho GTPase activation, cell adhesion, upregulation of α5 integrin, and F-actin polymerization in response to chemokines. In vivo experiments with Vav-driven Adam10-deficient mice show that the protease on hematopoietic cells is required for effective alveolar leukocyte recruitment during the early (24 hours) and later phase (72 hours) of LPS-induced acute pulmonary inflammation. At the later phase, ADAM10 deficiency correlates with decreased edema formation in response to LPS challenge. This was also observed in mice with LysM-driven Adam10 deficiency in myeloic cells. By contrast, ADAM17 is not required for leukocyte migration in vitro and in vivo. ADAM17 on leukocytes rather seems to mitigate migration, alveolar leukocyte accumulation, edema formation, and mediator release. However, these effects seem to be limited to the early phase of acute inflammation (24 hours), as ADAM17 deficiency had no effect on leukocyte recruitment and edema formation at a later stage of the inflammatory event.
This study demonstrates that pharmacological or genetic targeting of leukocyte-expressed ADAM10 reduces migration of human and murine monocytes and neutrophils in vitro and in vivo. This effect was correlated on the one hand with a reduction in adhesive properties to fibronectin and deficient upregulation of α5 integrin. The upregulation of α5 integrin by cytokines had been previously shown for TNF stimulation of tumor vascular leukocytes.22 This upregulation may be brought about via vesicular transport by microtubule motors such as kinesin family member 1C23 or other yet undefined mechanisms. The α5 integrin is part of the fibronectin receptor and required for adhesion and chemotaxis of monocytes,24 as well as for neutrophil and monocyte recruitment in LPS-induced pulmonary inflammation.25 In fact, α5β1 integrin blockage using RGDS peptides prevents lung damage induced by LPS.26 On the other hand, targeting of ADAM10 led to incomplete cell migration in vitro and in vivo as seen by an arrest of migrating cells in the chemotaxis membranes and within the lung tissue. Combining these 2 observations, it can be speculated that the reduced adhesion to matrix components such as fibronectin hinders cell migration and thereby prolongs the migration time through the chemotaxis membranes, through the endothelium, and through interstitial lung tissue, similar to the observed inhibition by RGDS peptides.26 Therefore, fewer cells would have completed migration, whereas the majority would still be in the progress of migration. Thus, ADAM10 deficiency leads to accumulation of slow-migrating cells in the chemotaxis membranes and interstitial accumulation within the lung. Interestingly, Mx-driven ADAM10 deficiency in mice leads to leukocyte accumulation in the spleen,27,28 and this effect could be in part due to the inability of leukocytes to pass through the spleen filter. Additionally, B cell-specific ADAM10 deficiency results in impaired marginal zone development and asthmatic responses,29,30 which might also depend on ADAM10-mediated B-cell migration.
In acute pulmonary inflammation, ADAM10 deficiency in all hematopoietic cells or preferentially in myeloic cells reduced recruitment of neutrophils and monocytes to the alveolar lumen. In line with the in vitro findings, the reduced ability of the cells to complete cell migration was associated with an accumulation of the infiltrating neutrophils and monocytes within the tissue. This may explain the observation why Vav-driven deficiency of ADAM10 does not protect against early changes in lung edema formation. Once activated, the trapped cells in the lung tissue including many neutrophils could cause destruction of the surrounding tissue, leading to increased permeability and edema formation. The fact, that lung tissue damage was not further enhanced could be due to reduced release of the neutrophil attracting chemokine CXCL1 and sIL6R in the BAL fluid, which would also limit further cell recruitment and tissue damage. At a later stage, however, Vav-driven or LysM-driven deficiency of ADAM10 conferred protection against LPS-induced lung damage. This may indicate that ADAM10-dependent long-lasting leukocyte recruitment contributes to maintain lung inflammation over a longer time period.
There are several possibilities of how ADAM10 can affect cell adhesion and migration. First, this seems to be a rapid and reversible mechanism, suggesting that potential effects of ADAM10 on cell differentiation do not play a role. This was confirmed with BMDMs in which ADAM10 did not affect the expression of the classical differentiation markers inducible nitric oxide synthase, CD206, and ICAM-1 or members of the Notch signaling pathway. One possible pathway may be indirect activation of ERK1/2 and p38 via ADAM10. We show that monocytic cell migration, adhesion to fibronectin, and upregulation of α5 integrin depend on both signals, and at least p38 activation requires the presence of ADAM10. It is known that ADAM10 and ADAM17 can induce shedding of EGFR ligands resulting in autocrine and paracrine EGFR transactivation,20 followed by ERK1/2 and p38 phosphorylation. This EGFR pathway enhances migration of endothelial and endometriotic cells.31,32 Importantly, we found that CCL2-induced α5 integrin upregulation and adhesion to fibronectin are not affected by EGFR inhibition. Although there may be a general effect of EGFR on cell migration, our data suggest that this is not the key molecule by which ADAM10 contributes to leukocyte recruitment and cell adhesion. Even though the responsible cleavage event by ADAM10 still needs to be determined, there obviously is an immediate effect of ADAM10 on the regulation of α5 integrin surface expression. Outside-in signaling by integrins is well known to mediate Rho GTPase activation and actin polymerization.21 In fact, we found that both responses are dependent on ADAM10. The hindrance of cytoskeletal rearrangement may then explain our observation that ADAM10-deficient leukocytes cannot efficiently transmigrate through the pores of the chemotaxis membranes in vitro and cannot efficiently transmigrate through the lung tissue in vivo.
In comparison with ADAM10, the closely related protease ADAM17 seems to hold a different role in cell migration. By conditional knockout of ADAM17 in hematopoietic cells or adoptive transfer of ADAM17-deficient bone marrow, we and others consistently found increased neutrophil recruitment in the early phase of the inflammatory response when leukocytes lack ADAM17.9,33,34 This increased presence of leukocytes would lead to enhanced tissue damage and may thus explain the increased edema formation observed in mice lacking leukocyte ADAM17. ADAM17 but not ADAM10 mediates l-selectin shedding, and loss of l-selectin on the surface of neutrophils would lead to enhanced rolling and adhesion to the endothelium,35 which would then promote inflammatory recruitment of neutrophils.9,36 In fact, loss of l-selectin shedding by ADAM17 has been correlated with reduced pulmonary recruitment of neutrophils.31 A recent study reported a crucial role of ADAM17 in monocyte transendothelial migration but not in endothelial adhesion. This was associated with surface regulation of macrophage adhesion ligand-1 (MAC-1) (αMβ2 integrin), which was ADAM17 but not ADAM10 dependent.36 We confirm that ADAM10 has no influence on chemokine-induced upregulation of the MAC-1 components β2 integrin and αM integrin, and we also did not observe an effect on leukocyte function-associated antigen 1 (LFA-1) (αLβ2 integrin). However, we found prominent effects of ADAM10 on chemokine-induced upregulation of α5 integrin in both neutrophils and monocytes. Although MAC-1 and LFA-1 are involved in leukocyte interactions with endothelium, α5 integrin is part of the fibronectin receptor (α5β1) and therefore important for cell-matrix interaction.37 Thus, ADAM17 and ADAM10 could play different roles in the interaction of leukocytes with endothelial cells and extracellular matrix components.
There is increasing evidence that metalloproteinase inhibitors can limit hallmarks of acute pulmonary inflammation.12,38 This is most likely a result of distinct metalloproteinase activities on multiple cell types. For ADAM17, we recently reported that the protease on endothelial cells is proinflammatory in a murine model of acute pulmonary inflammation.12 However, this activity may be counteracted at least in part by ADAM17 on leukocytes, limiting neutrophil migration as reported in the present study and by others.9,33,34 The role of ADAM10 is apparently different because its proinflammatory activities on endothelial cells may synergize with its proinflammatory activities on leukocytes that have been identified in the present study.12,13,39 Thus, local treatment with selective ADAM10 inhibitors may represent a novel strategy to limit pulmonary inflammation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Carl Blobel (Sloan Kettering, NY), and Keisuke Horiuchi (University of Tokyo) for providing Adam17flox/flox, and Bart de Strooper (University of Leuven) for providing Adam10flox/flox mice. The authors also thank T. Woopen and M. Esser for technical assistance.
This work was supported in part by the Interdisciplinary Center for Clinical Research (IZKF) Aachen (A.L.) and the Deutsche Forschungsgesellschaft (DFG) grants Lu869/5-1 (A.L.) and SFB877-A3 (P.S.).
Authorship
Contribution: J.P., F.M.H., H.A., E.G., T.P., S.N., J.K., E.v.d.V., and D.D. performed experiments and analyzed data; A.L., D.D., J.P., and F.M.H. wrote the manuscript and designed the study; and C.M., S.U., M.D., and P.S. provided vital expertise, the animal model, or transgenic mice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniela Dreymueller, Institute of Pharmacology and Toxicology, Medical Faculty RWTH Aachen University, Pauwelsstrasse 30, 52074 Aachen, Germany; e-mail: ddreymueller@ukaachen.de.
References
Author notes
J.P., F.M.H., D.D., and A.L. contributed equally to this work.

![Figure 1. Effect of metalloproteinase inhibitors and ADAM10/ADAM17 knockdown on THP-1 cell migration. (A-B) THP-1 cells were preincubated with varying concentrations of the inhibitors (A) GI254023X (GI) in or (B) TAPI-1 or appropriate dilutions of vehicle (dimethylsulfoxide [DMSO]) for 15 minutes and then assayed for cell migration in response to 3 nM CCL2. The number of migrated cells in the lower compartment was determined by measurement of endogenous glucuronidase activity, and the results are expressed in relation to cells receiving no chemoattractant and no inhibitor. Migration of cells receiving no chemoattractant is indicated by dotted lines. (C) THP-1 cells were pretreated with GI254023X (10 µM) or DMSO (0.1%) for 30 minutes. Subsequently, cells were either directly assayed for CCL2-induced cell migration or washed to remove free inhibitor. Washed cells were either directly investigated for CCL2-induced chemotaxis or further incubated for 60 minutes (recovery) before the assay. Results are expressed in relation to the untreated control receiving no chemoattractant (dotted line). (D) THP-1 cells were transduced with different lentivirus encoding scramble-shRNA (scr), ADAM10-shRNA (A10-650 and A10-1947), or ADAM17-shRNA (A17-2061 and A17-2646) and assayed for CCL2-induced cell migration. Results are expressed in relation to the scramble control receiving no chemoattractant (dotted line). (E) Following CCL2 stimulation in the presence of 10 µM GI254023X or 0.1% DMSO (15-minute pretreatment), cells were removed from the top of the chemotaxis membrane, and the remaining cells were stained in the chemotaxis membrane. (Left) Representative images and (right) quantitative data are shown. (F) Green fluorescent protein-expressing THP-1 cells were pretreated with 10 µM GI254023X or 0.1% DMSO and subsequently investigated for transmigration through dsRed-expressing ECV304 cells grown on transwell filters. THP-1 cells were (left) visualized by confocal microscopy and Z-stack analysis and (right) quantified within (pos.1) and below (pos.2) the ECV304 layer. Quantitative data represent means ± SEM of 3 independent experiments. Significance was calculated using 1-way ANOVA and the Bonferroni post-test and is indicated by crosses. Asterisks without a line indicate significant differences to the nontreated control analyzed by 1-sample t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/26/10.1182_blood-2013-09-511543/4/m_4077f1.jpeg?Expires=1769430090&Signature=E~FhD5Ar-vWUj2P8IbCHQAlagAsygk-vwvjUtqy1Dene8K7VoL6L3TgVgk~Rtw4fwqrHFsgA7lNJcIFejonA9Yj6hkLudrWdKQL5okXtEgTYS1kvz1vW5v3U954hOtsXW6u33snIN4B5-PRJLWltTnVZpBMxrytTWXrCb290jr8v2JXjAPcyC7kVB7FiIohtRWnmyMnSTIJAbHGGsYzaSPEP81XetNjxBuan5pA~06sAkjtGB7kKJaKO47ZPAUZ7ddXgCKHreo42161qQIJh4a3jD2pab0p0bC8CmfQvW~ClvrfjvB7RLw14pj8i7ItFKst5hBJ69lvn0ea0iS4I6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal