In this issue of Blood, Tidwell et al1 demonstrate that mutations in the start codon (protein synthesis is initiated at the codon ATG) of neutrophil elastase (ELANE) result in the production of N-terminally truncated elastase, which mislocates to the nucleus and results in severe congenital neutropenia (SCN).
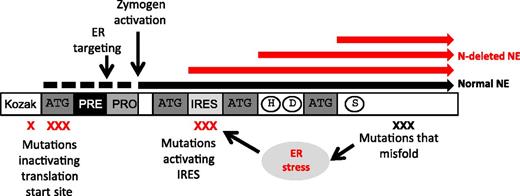
Mutations in the ATG site normally used as start of translation and other mutations, as indicated by the red X, result in the production of N-terminally truncated elastase lacking the signal peptide (red bars). These truncated proteins are liberated to the cytosol instead of being routed to the endoplasmic reticulum and granules as is wild-type elastase (black bar). See Figure 6 in the article by Tidwell et al that begins on page 562. ER, endoplasmic.
Mutations in the ATG site normally used as start of translation and other mutations, as indicated by the red X, result in the production of N-terminally truncated elastase lacking the signal peptide (red bars). These truncated proteins are liberated to the cytosol instead of being routed to the endoplasmic reticulum and granules as is wild-type elastase (black bar). See Figure 6 in the article by Tidwell et al that begins on page 562. ER, endoplasmic.
SCN is a rare condition with a serious impact on heath and quality of life. Although treatment with granulocyte colony-stimulating factor (G-CSF) largely restores circulating neutrophil counts and effectively reduces infections, the condition carries a risk of progression to acute myeloid leukemia of 2.3% per 10 years.2
A major breakthrough in understanding the genetic background of SCN and its more benign relative, cyclic neutropenia, was the discovery by Marshall Horwitz and David Dale and colleagues that virtually all forms of cyclic neutropenia, and most forms of autosomal-dominant SCN, which covers about 70% of the cases, are caused by mutations in the coding region of ELANE, the gene for neutrophil elastase,3,4 one of the 4 serine proteases localized to azurophil granules of neutrophils along with the commonly used neutrophil marker, myeloperoxidase. These seminal papers were presented before the era of exome sequencing and were the result of meticulous studies of pedigrees of patients that made it possible to narrow down the gene defect to chromosome 19p13.3 and then verify that mutations were present in ELANE.
Why should mutations in the gene for elastase cause such problems? Although it is still not evident why ELANE mutations result in cyclic neutropenia, it is more easily comprehended that mutations in ELANE, one of the genes most abundantly expressed in promyelocytes,5 may perturb the further development of these cells when transcription of such mutated ELANE is at its peak: but how? Several mechanisms have been offered. One explains elastase as a transmembrane protein with SCN mutations preventing its sorting to granules, resulting in routing to the plasma membrane, where it may cause havoc.6 Another argues that mutations result in misfolding of elastase, which exceeds the capacity of the endoplasmic reticulum for corrections and induces an unfolded protein response leading to death of the cells,7 much akin to the necrosis of liver cells in severe forms of α-1–antitrypsin deficiency.8 Why G-CSF should ameliorate this is not quite evident, but it is perhaps the result of reduction in production time and hence the amount of protein synthesized at the promyelocyte stage before other transcription factors take over and shut down the production of the offending misfolded protein.
In this issue of Blood, a novel mechanism is put forward and supported by several lines of evidence.1 The authors identified 8 cases of SCN where the mutations of ELANE affect 1 of the 3 nucleotides of the canonical translation initiation codon, ATG, which puts methionine on as the first amino acid when protein synthesis starts in all species. When this is mutated, the translational machinery will skip this site for initiation of translation and search for alternative ATG sites further downstream that satisfy the minimal requirements for initiation of translation, and such are indeed present in ELANE but alas result in shortened forms of elastase (see figure). Importantly, the part that is skipped codes for the signal peptide that guides proteins into the endoplasmic reticulum and eventually into granules. Instead, these N-terminally truncated forms are produced as cytosolic proteins. The presence of active proteases in the cytosol could certainly be expected to elicit apoptosis, but these truncated elastase forms are barely active and do not induce apoptosis. Instead, they stick to the nuclei of the cells. Although the authors demonstrate that such mutations negatively affect the proliferation of cells, it is still an open question whether the truncated forms by virtue of their mislocation and charge can block access of transcription factors to the nucleus necessary for further differentiation and hence explain the block of differentiation so characteristic of this condition.
These mutations are found only in a small minority of patients, but other ELANE mutations, located in the vicinity of the alternative translation initiations sites, make these more palatable for ribosomes to start translation at such internal ribosomal entry sites and putatively result in truncated elastase and SCN, even when the traditional start site is not mutated.
Although this paper concerns a small fraction of patients with a rare disorder, the study opens a new path for understanding how genetic defects affect cellular differentiation that may be relevant to other diseases, both congenital and acquired, not the least of which include the myelodysplastic syndromes and acute myeloid leukemia.
Conflict-of-interest disclosure: The author declares no competing financial interests.