In this issue of Blood, Steinberg et al describe the clinical importance of the distribution or “packaging” of fetal hemoglobin (HbF) within erythrocytes of persons with sickle cell anemia.1
Disease phenotype depends on how HbF is packaged in HbS-containing erythrocytes.
Disease phenotype depends on how HbF is packaged in HbS-containing erythrocytes.
Ever since Janet Watson’s astute observation in 1948,2 the “protective” effect of HbF in persons with sickle cell anemia has been appreciated, and during the last several decades, increasing HbF levels by prescribing hydroxyurea has had salutary clinical effects. HbF has been measured by its overall concentration in the blood or by the percentage of erythrocytes containing fetal hemoglobin (F cells).3 Now, Steinberg et al elegantly characterize a previously unappreciated third means of expressing the powerful ameliorative effects of HbF in patients with sickle cell anemia: ie, the percentage of erythrocytes containing ≥10 pg of HbF. This amount has been shown to inhibit deoxy sickle hemoglobin (HbS) polymer formation when the oxygen saturation is less than the 40% to 70% encountered in the microcirculation. By preventing polymerization, the beneficial effects for the patient include reduction in intravascular hemolysis, vaso-occlusion–induced pain and tissue damage, and endothelial injury.
The authors note the experiment of nature exemplified by the phenotype resulting from the compound heterozygous state of HbS and pancellular hereditary persistence of fetal hemoglobin (S-HPFH).4 In this condition, every erythrocyte remarkably contains not only HbS but also approximately 10 pg of HbF. The result is nearly complete inhibition of deoxyHbS polymerization so that such individuals have a normal phenotype or only very minimal hemolysis. The authors thus posit that a therapeutic goal of achieving 10 pg of HbF per cell in persons with sickle cell anemia could represent a pharmacologic cure.
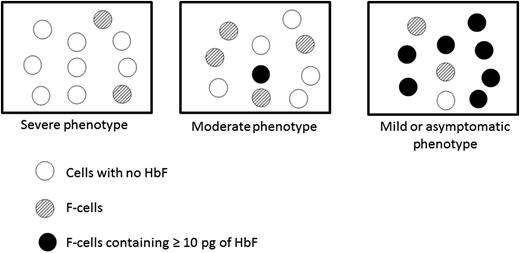
Hydroxyurea therapy is often beneficial (and correlates roughly with the percentage rise in HbF), but unfortunately the deleterious clinical manifestations of intravascular hemolysis and vaso-occlusion frequently continue.5 In this article, the authors describe examples from the clinical sickle cell literature of diverse β globin haplotypes that are associated with widely ranging HbF values and clinical sequelae. Yet, because it is uncommon for HbF levels to exceed 20% to 25% (with or without hydroxyurea), most persons with sickle cell anemia have pain, organ damage, and a shortened lifespan. Nevertheless, the authors depict in various hypothetical examples how persons with specific concentrations of HbF or percentages of F cells can have widely differing percentages of circulating erythrocytes that exceed the protective threshold of 10 pg/cell, ranging from virtually none to 70% or more (see figure).
A clinical correlate of the observation by Steinberg et al is the use of a chronic hypertransfusion program for primary or secondary stroke prevention or as a means of reducing the frequency or severity of vaso-occlusive crisis and acute chest syndrome. Suppressing the proportion of cells containing HbS to <30%, a common goal of chronic transfusions, assures that sickle cell–related complications are reduced or prevented.6 The result would be similar when 70% HbS-containing cells also have >10 pg of HbF/cell (as in persons with HbS-HPFH).
So how do we achieve the postulated objective of increasing HbF to >10 pg in most or nearly all of these patients’ cells? Clearly hydroxyurea alone is not capable of that goal without risks of excessive toxicity. The authors point out that even the promising approach of blocking the BCL11A pathway7 may not result in the desired pharmacologic cure unless the result is a pancellular increase in HbF as observed in compound heterozygotes with HbS-HPFH.
The authors’ characterization of packaging HbF in the majority of cells to achieve a 10-pg level and render them incapable of sickling is an admirable goal. What an achievement it would be if each individual in the world with sickle cell anemia could someday be presented with such a therapeutic package as a gift of life.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal