Abstract
Despite the damaging effect on tissues at a high concentration, it has been gradually established that oxidative stress plays a positive role during angiogenesis. In adults, physiological or pathological angiogenesis is initiated by tissue demands for oxygen and nutrients, resulting in a hypoxia/reoxygenation cycle, which, in turn promotes the formation of reactive oxygen species (ROS). The ROS can be generated either endogenously, through mitochondrial electron transport chain reactions and nicotinamide adenine dinucleotide phosphate oxidase, or exogenously, resulting from exposure to environmental agents, such as ultraviolet or ionizing radiation. In many conditions, ROS promotes angiogenesis, either directly or via the generation of active oxidation products, including peroxidized lipids. The latter lipid metabolites are generated in excess during atherosclerosis, thereby linking atherogenic processes and pathological angiogenesis. Although the main mechanism of oxidative stress-induced angiogenesis involves hypoxia-inducible factor/vascular endothelial growth factor (VEGF) signaling, recent studies have identified several pathways that are VEGF-independent. This review aims to provide a summary of the past and present views on the role of oxidative stress as a mediator and modulator of angiogenesis, and to highlight newly identified mechanisms.
Introduction
Angiogenesis is defined as the process of sprouting new blood vessels from preexisting vasculature. New blood vessel formation is essentially required for many physiological processes, such as embryogenesis, tissue repair, and organ regeneration.1 This process, however, needs to be finely balanced, because excessive or insufficient angiogenesis contributes to a number of pathologies, ranging from cancer, macular degeneration, and retinopathy of prematurity to impaired repair of ischemic tissues.2 Angiogenesis is a truly systemic process that requires the responses of multiple cell types, including endothelial, mural, inflammatory, and blood-derived cells.3 These cells participate in a range of processes, such as cell adhesion, migration, proliferation, and differentiation, thereby adding another level of complexity.4 Angiogenesis, either physiological or pathological, requires initiation by proangiogenic factors, exemplified by vascular endothelial growth factor (VEGF), placental growth factor, platelet-derived growth factor-B, transforming growth factor β, and angiopoietin-1 (ANG-1).2 In most situations, if not all, angiogenesis is closely interwoven with the mobilization of inflammatory cells.5 During physiological or repair processes, such as wound healing, the inflammation process is transient; most pathological conditions, exemplified by cancer, involve a continuous recruitment of inflammatory cells, which, in turn, serve as a substantial source of ROS.6 This functional connection between the inflammation-dependent generation of ROS and angiogenesis plays an important role during various stages of tumor progression, from its initiation stage to vascularization and metastasis. Moreover, in most pathologies, oxidative stress operates as part of a positive feedback mechanism, which gives it even more signification in the process.7
Oxidative stress, which is defined as an imbalance between prooxidant and antioxidant systems,7 can be both a cause and consequence of many vascular complications and serve as one of the biomarkers for these conditions. At the same time, well-controlled oxidative stress may be beneficial for angiogenesis during tissue repair. In this review, we summarize the history and recent findings on the relationship between oxidative stress and angiogenesis, and discuss the implications of oxidative stress on pathological conditions and therapeutic strategies.
ROS generation and accumulation
Chemistry of oxidative stress
By 1 electron at a time, oxygen can be sequentially reduced to 4 components: superoxide anion, hydrogen peroxide, hydroxyl radical, and a water molecule.8 During this reduction-oxidation (redox) reaction, ROS are produced as intermediates in vivo. Superoxide anion is known to be a main contributor to the generation of most ROS and a crucial mediator of electron transport chain reactions in mitochondria. Usually, superoxide anion is rapidly removed through dismutation to hydrogen peroxide, either spontaneously or by superoxide dismutases (SOD).8,9 Neutrophil-secreted myeloperoxidase further converts hydrogen peroxide and chloride into highly reactive hypochlorite. For vascular cells, superoxide anion and hydrogen peroxide appear to be particularly important because they are able to activate diverse pathways to induce either new vascular growth, or vascular dysfunction and destruction.10
ROS can be generated by all vascular cell types, including endothelial cells, smooth muscle cells, adventitial fibroblasts, and perivascular adipocytes.11 There are 2 main endogenous sources in the vasculature: mitochondrial electron transport chain reactions and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.11-13 In mitochondria, more than 95% of oxygen consumed by cells is used to yield water molecules through redox reactions.14 Particularly, at complex I and III in the transport chain, premature electron leakage to oxygen occurs, which causes less than 4% of oxygen to be reduced to superoxide anion, but not to water, generating oxidative stress.8,10 NADPH oxidase, an enzyme that generates superoxide anion by transferring electrons from NADPH to oxygen, is recognized as a major source of ROS in many cell types, including endothelial and smooth muscle cells.10,13-17 It is important to note that in many conditions, the respiratory (oxidative) burst of inflammatory cells, such as neutrophils and monocytes, is the main contributing factor to ROS levels in a number of vascular pathologies. For example, myeloperoxidase is believed to be one of the main players in vasculitis and coronary artery disease.18
One of the main consequences of ROS presence is peroxidation of proteins and lipids, resulting in their functional modifications.19,20 In vivo, lipid peroxidation affects cellular membrane lipids, including the polyunsaturated fatty acid (PUFA) part of phospholipids, which are particularly prone to oxidation, generating a new array of biologically active agents.19 These oxidation-generated secondary compounds may be even more destructive than ROS themselves.20 In sum, oxidative stress entails not only the production and accumulation of ROS, but also oxidative stress-induced metabolic products generated through lipid and protein peroxidation.
Oxidative stress as mediator of angiogenesis
ROS act as a double-edged sword in the vasculature because chronically produced or highly concentrated ROS are detrimental for most tissues, whereas transient or low levels of ROS are able to activate signaling pathways that eventually promote regeneration and growth.21,22 Thus, ROS are implicated, either directly or indirectly, in the process of physiological, pathological, and excessive angiogenesis.
One of the early implications of ROS in angiogenesis resulted from the studies using thiol-containing compounds, which were shown to inhibit macrophage-derived proangiogenic activity, as the conditioned medium from gold sodium thiomalate-treated macrophages potently reduced angiogenesis in rat corneas.23,24 Although an effect was somewhat indirect, the follow-up studies from the same group demonstrated that thiol-containing compounds acting as ROS scavengers may inhibit production of angiogenic factors by macrophages.23-25
A number of studies using hydrogen peroxide have concluded that at a high concentration, hydrogen peroxide causes endothelial injury; however, at a low concentration, it generally stimulates angiogenesis, as hydrogen peroxide >125 μM induces lethal vascular endothelial cell injury, but between 0.1 and 10 μM, the tube formation was obtained in the endothelial cells from the bovine thoracic aorta.26-28 It has been suggested that tumor angiogenesis is triggered by hydrogen peroxides, which are produced in large amounts by tumor cells themselves.28,29
Several studies have shown that conditions of hypoxia followed by reoxygenation, but not hypoxia alone, accelerate tubular morphogenesis in human microvascular endothelial cells and promote myocardial angiogenesis in animal models of chronic myocardial infarction.30,31 In human umbilical vein endothelial cells (HUVECs), leptin increases the accumulation of ROS, thereby promoting angiogenesis.32
These conclusions are further supported by studies using oxidase inhibitors and general antioxidants. NADPH oxidase inhibitors, such as diphenyliodonium and apocynin, as well as free radical scavengers, such as mannitol and catalase, appear to inhibit angiogenesis.25,33 A similar effect has been observed in studies using a potent ROS scavenger, N-acetylcysteine.34 Adenoviral overexpression of extracellular SOD inhibited tumor vascularization and growth melanoma.35 Thus, the antiangiogenic effects of antioxidants have further confirmed the overall proangiogenic function of ROS.
This notion is further supported by mouse genetic studies. The vascular remodeling induced by ANG-1 is more prominent in catalase knockout mice than in wild-type controls.36 Nox1 knockout mice, lacking a component of NADPH oxidase, have shown a reduced endothelial cell migration and reduced formation of tube-like structures.37 Likewise, a lack of NADPH oxidase 2 (Nox2) substantially impairs VEGF-induced angiogenesis and neovascularization in the ischemic hindlimb model.38,39 ANG-1-stimulated ROS, endothelial cell migration, and sprouting from aortic rings are suppressed in the mice that are deficient in p47phox, another component of NADPH oxidase.40
Mechanisms of oxidative stress-induced angiogenesis
Oxidative stress, oxidized products, and VEGF signaling
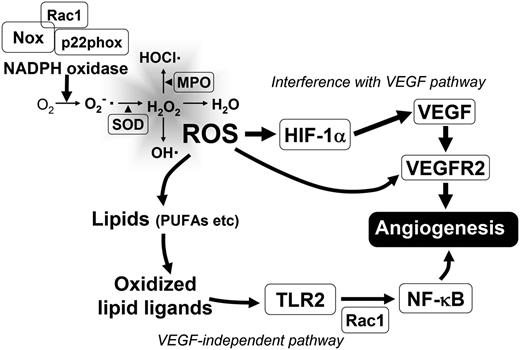
VEGF is the most potent and primary endothelial specific angiogenic growth factor, both in physiological and pathological conditions. VEGF signaling is ultimately required for normal vascular development and homeostasis, but it is also actively engaged in tumor progression by promoting growth of tumor vasculature.41 This signaling pathway often seems to be affected by ROS. Exogenous ROS stimulate the induction of VEGF expression in various cell types, such as endothelial cells, smooth muscle cells, and macrophages, whereas VEGF induces endothelial cell migration and proliferation through an increase of intracellular ROS.38,42-45 Thus, the reciprocity between oxidative stress and angiogenesis has been centered on the VEGF signaling pathway (Figure 1).10 A number of studies have demonstrated this positive interrelationship between ROS and angiogenesis. Hydrogen peroxide induces VEGF expression in vascular smooth muscle cells, as well as endothelial cells, and thereby promotes angiogenic responses.42,44 In several pathologies, exemplified by diabetic retinopathy and injured arteries, ROS-mediated angiogenesis is strongly associated with VEGF expression.42,46,47 At the same time, VEGF further stimulates ROS production through the activation of NADPH oxidase in endothelial cells.12 VEGF-induced superoxide anion production is regulated by components of NADPH oxidase, including Rac1, Nox1, and Nox2, as it is significantly inhibited in HUVECs by either overexpression of dominant negative Rac1 or transfection of Nox2 antisense oligonucleotides.38 In addition, overexpression of Nox1 increases VEGF expression in NIH3T3 fibroblast and DU-145 prostate cancer cells, and VEGF receptor (VEGFR) 2 expression in endothelial cells of tumor blood vessels, through ROS production.48 ROS also affect VEGF-stimulated VEGFR2 dimerization and autophosphorylation, which are required for VEGFR2 activation and subsequent angiogenesis (Figure 1).38,49 Likewise, products of oxidation exemplified by oxidized phospholipids (OxPLs) are reported to interact with VEGFR2, and thereby stimulate angiogenesis via Src-mediated signaling.50 Thus, ROS promote angiogenic responses in various tissues by operating both upstream and downstream of VEGF/VEGFR2 signaling.
Schematic representation of ROS generation and its effect on angiogenesis. Two main mechanisms are shown: ROS effect on known components of HIF-VEGF/VEGFR2 signaling pathway and VEGF-independent mechanism involving generation of lipid oxidation products. MPO, myeloperoxidase.
Schematic representation of ROS generation and its effect on angiogenesis. Two main mechanisms are shown: ROS effect on known components of HIF-VEGF/VEGFR2 signaling pathway and VEGF-independent mechanism involving generation of lipid oxidation products. MPO, myeloperoxidase.
There are a number of compelling studies reporting the augmentation of angiogenesis by oxidation products (eg, oxidized lipids, which often operate via the hypoxia-inducible factor (HIF)/VEGF pathway). OxPLs have been found to stimulate endothelial cell sprouting in vitro and promote capillary growth in vivo.51 OxPLs also stimulate VEGF expression in vivo, as well as in several cell lines in vitro, as oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine increases VEGF messenger RNA expression in HUVECs, human adult skin fibroblasts, primary human bronchial epithelial cells, human and primary mouse keratinocytes, and epithelial tumor cell lines of different tissue origin, such as MCF-7, Hep3B, and HeLa.51 Particularly, oxidized low density lipoproteins (oxLDL), which are generated by oxidative stress also increase VEGF expression and its messenger RNA stability in human macrophages.52 OxLDL also strongly induces HIF-1α, as well as VEGF expression in monocyte-macrophages and significantly increases tube formation in cocultured endothelial cells.53,54 HIF-1α inhibition abrogates the proangiogenic effect of oxLDL in vivo, suggesting that the HIF-1α pathway is a possible molecular mechanism by which oxLDL induces angiogenesis.53
VEGF-independent mechanisms of oxidation-induced angiogenesis
Several recent studies have identified new mechanisms of ROS-activated angiogenesis that operate in a VEGF-independent manner. One of these mechanisms mediating proangiogenic function of ROS involves the generation of new lipid oxidation products with proangiogenic activities.55,56 One of the PUFA of phospholipids, docosahexaenoic acid (DHA), is recognized as a highly abundant and oxidation prone substrate in many human tissues, especially in the retina and brain.19,57 PUFA-derived γ-hydroxyalkenal oxidized phospholipids are known to produce ω-carboxyalkylpyrrole (CAP) modifications of proteins after the lipolysis of intermediate phospholipid adducts.58 As such, ω-carboxyethylpyrrole (CEP) protein adducts are generated from the oxidation of 1-palmityl-2-docosahexanoyl-sn-glycerophosphocholine.58 As the end product of DHA oxidation, CEP serves as an important biomarker for oxidative stress-induced vascular pathologies in retina, such as age-related macular degeneration.CEP is generated in retinas with age-related macular degeneration, during wound healing, and also in highly vascularized tumors, where it promotes angiogenesis.58-60 The neutralization of endogenous CEP in mice by a CEP-specific antibody impairs wound healing, tissue revascularization, and diminishes tumor angiogenesis, thereby demonstrating the key role of this pathway in various models of angiogenesis.59 Importantly, the molecular mechanism by which CEP induces angiogenesis is found to be independent on VEGF/VEGFR2 and operates via activation of the Toll-like receptor (TLR) 2/MyD88 pathway.59 This leads to the activation of Rac1 to promote cell migration and angiogenesis.59 Curiously, Rac1 is a component of NADPH oxidase, suggesting that in certain conditions, this pathway may be interconnected with VEGF-mediated or NADPH oxidase-mediated signaling in the angiogenic network (Figure 1).61
Likewise, other studies have convincingly shown that TLRs play important roles beyond innate immunity and function as significant contributors to angiogenesis.62 To date, several proangiogenic ligands of TLRs, such as lipopolysaccharide as a TLR4 ligand, poly(I:C) as a TLR3 ligand, macrophage-activating lipopeptide-2 as a TLR2/6 ligand, and CEP as a TLR2 ligand, were generated by oxidative stress.63-68 Another recent study reports that peroxidized unsaturated fatty acids stimulate TLR4 signaling on endothelial cells, leading to nuclear factor κB activation, as TLR4 was acutely translocated to caveolae/raft membranes upon treatment.69 Considering the angiogenic activity of lipopolysaccharide/TLR4 signaling, this peroxidized unsaturated fatty acids-mediated TLR4 activation may have an effect on angiogenesis in vivo. TLR signaling has also been shown to affect the HIF/VEGF pathway of angiogenesis. TLR2 has been shown to modulate the expression of HIF-1α, which activates the VEGF promoter in response to hypoxia (Figure 1).70
Another comprehensive study has connected oxidative stress and VEGF-independent angiogenesis, and has identified a new function of ataxia-telangiectasia mutated (ATM) kinase in oxidative defense.71 ATM is selectively activated in immature blood vessels in response to ROS accumulation.71 Unlike CEP-TLR2 mediated angiogenesis, ATM-induced angiogenesis is only limited to promoting pathological responses, such as excessive tumor angiogenesis.71 ATM promotes the proliferation of endothelial cells by suppressing high levels of ROS. The downstream of the ROS-induced ATM activation pathway is identified as p38α, as the increased ROS by ATM deficiency activate p38 both in vitro and in vivo.71 Importantly, ATM deficiency synergistically enhances the antiangiogenic effect of VEGF inhibition in mouse tumor growth and angiogenesis by SU1498, a specific VEGFR2 inhibitor, indicating a VEGF-independent proangiogenic role of ATM.71
Oxidative stress induced-angiogenesis in pathologies
Angiogenic processes influenced by oxidative stress
Angiogenesis represents several interwoven components, including endothelial responses and the mobilization of proangiogenic bone marrow (BM)-derived cells; oxidative signaling has been found to modulate all of these processes.72 The release of proangiogenic cells from the BM has been shown to be dependent on ROS signaling, as Nox2 knockout mice exhibited impaired ischemia-induced blood flow recovery, which is linked to ROS levels in BM mononuclear cells and their mobilization from the BM.73 Intracellular ROS production mediated by NADPH components Nox2 and Nox4 promotes proliferation and survival of endothelial cells via p38, extracellular signal regulated kinase, and Akt signaling.74-78 A scaffolding protein IQGAP1 appears to be involved in the different stages of angiogenic processes, including ROS-mediated disruption of VE-cadherin–containing adherence junctions and VEGF2 signaling, both of which are known to promote endothelial migration.79,80
Physiological angiogenesis by oxidative stress
Physiological angiogenesis in adult organisms is required for tissue repair and remodeling processes, such as wound healing, skeletal remodeling, and female reproduction.2 In wound healing, angiogenesis is induced by tissue hypoxia and ROS, which either stimulate macrophages, fibroblasts, endothelial cells, and keratinocytes to produce VEGF,6,43,81-83 or operate in a VEGF-independent fashion.59 During endochondral ossification during longitudinal bone growth, increasing ROS promote chondrocyte hypertrophy, which, in turn, induces angiogenesis. Antioxidant treatment (eg, by N-acetylcysteine) inhibits these processes.84 Angiogenesis is important for cyclical regeneration of endometrium in the menstrual cycle and is also regulated by ROS.85 SOD expression shows a cyclical variation in the endometrium, and its activity decreases in the late secretory phase, whereas lipid peroxide levels increase.86 Another study has reported that the vascular density is significantly increased in the late secretory phase as compared with the late proliferative phase, supporting the timely requirement of ROS on the angiogenic potential of endometrium.87
Pathological angiogenesis by oxidative stress
Pathological angiogenesis is fundamentally similar to physiological angiogenesis and aims to promote new blood vessels to supply oxygen and nutrients. However, pathological angiogenesis proceeds in an uncontrolled and unbalanced manner, resulting in an excessive and abnormal vascular pattern.2 In most pathologies ranging from tumorigenesis, eye diseases, and atherosclerosis, angiogenesis is greatly affected by oxidative stress.2,16,88 In tumors, both tumor and stromal cells, including inflammatory cells, produce substantial amounts of ROS.29,89,90 Nox1-induced ROS production activates oncogenic Ras signaling and promotes upregulation of VEGF, which, in turn, augments tumor angiogenesis.91 In prostate cancer, NADPH oxidase-derived ROS causes HIF-1α activation and the subsequent release of VEGF.92 Often, inhibition of both VEGF and oxidative pathways produces a synergistic effect on angiogenesis.59
In retinopathies, where abnormal angiogenesis is regarded as a hallmark and a cause of pathology,2 ROS production is highly elevated due to high oxygen tension and insufficient ROS scavenging by antioxidants, such as vitamins C and E and copper/zinc SOD.93-95 As a result, superoxide anion and hydrogen peroxide levels have shown to be substantially elevated in animal models of diabetic retinopathy or retinopathy of prematurity.96 Even normal retinas seem to be particularly sensitive to oxidative stress, due to a high consumption of oxygen, high levels of oxidation substrates (eg, PUFAs), and exposure to light.97 DHA, one of the easily oxidizable PUFAs, represents 60% of the PUFAs in the retina57 and the product of its oxidation serves as a biomarker for oxidative stress-induced injury in age-related macular degeneration.60,98 Likewise, ischemic retinopathy is substantially diminished in ATM-deficient mice in which excessive ROS are inhibited.71
Pathological angiogenesis promotes atherogenic processes by increasing both macrophage infiltration and the thickening of blood vessel wall.12 Similar to other pathologies, this angiogenesis is highly and directly augmented by ROS and ROS products; this is represented by metabolites of lipid oxidation, which are generated and accumulated in plasma and the vessel wall of both mice and humans.51,99-101
Therapeutic implications
As a master regulator for angiogenesis, VEGF and its pathway have served as primary targets for antiangiogenic therapy.102-103 The current view on the anti-VEGF therapy, using an anti-VEGF antibody or VEGFR inhibitors, is not as optimistic as it was several years ago.104 Despite rather disappointing results of recent clinical studies, drug targeting of the angiogenic process stimulated and accelerated the development of alternative antiangiogenic approaches.103-104 Moreover, pathological angiogenesis shares many pathways, most notably VEGF/VEGFR2, with physiological processes, thus hindering both the success and safety of anti-VEGF therapy.105 Despite the great importance of VEGF during oxidative stress-induced angiogenesis, there are a variety of VEGF-independent signaling pathways implicated in pathological angiogenesis, such as the ROS/ATM/p38α and CEP/TLR2/MyD88 pathway, which may reduce the efficacy of anti-VEGF therapy.55 At the same time, inhibition of VEGF pathway may disturb the homeostatic maintenance of normal vasculature and result in delayed wound healing, hypertension, proteinuria, thrombosis, and hemorrhage.105-107 Thus, there is a need for a specific target discriminating pathological vasculature from a healthy one. In this regard, it is noteworthy that ATM is selectively activated in immature pathological blood vessels, but not in normal vessels, in response to ROS, suggesting the potential of anti-ATM therapy.71
In most pathologies, it may be beneficial to target oxidative stress, per se. One possibility is the use of pharmacological inhibitors of NADPH oxidase, which is the main source of ROS in vasculature.108-109 However, the studies using such inhibitors in vivo animal models15,16 are limited. Another strategy to diminish oxidative stress-induced pathological angiogenesis is the use dietary antioxidants, which also may prevent vascular pathologies.110 For instance, vitamins C and E reduce VEGF and VEGFR2 expression in Apolipoprotein E knockout mice.111 Both red wine polyphenolic compounds and green tea polyphenols inhibit angiogenesis by reducing the expression of VEGF and matrix metalloproteinase-2.112 Similarly, resveratrol, a natural compound found in grapes and red wine, suppresses angiogenesis, significantly inhibits fibrosarcoma growth, and delays angiogenesis-dependent wound healing in mice.113 However, most of these antioxidants are nonspecific and affect both pathological and physiological angiogenesis. Furthermore, it is difficult to determine the optimal dose of the inhibitor for each pathological condition in each individual, resulting in additional limitations of anti-VEGF therapy, NADPH oxidase inhibitors, or general antioxidants. Therefore, there is a need for a better understanding of the precise molecular mechanisms behind oxidative stress-induced angiogenesis and the identification of markers and processes specific for either pathological or physiological angiogenesis.
Conclusions
As in the drawings of Maurits Escher, multiple components of angiogenesis are interlinked with oxidative stress. The former process cannot be regarded simply as either friend or foe because it appears to have different and often opposite functions, similar to snake venoms, which can either deliver an antidote or result in severe damage in excessive quantities.
Physiologically appropriate levels of ROS, generated either in restricted amounts or in a transient fashion, are essentially required to promote physiological angiogenesis and homeostatic maintenance of healthy vasculature. However, uncontrolled continuous ROS production will ultimately contribute to pathology and cause tissue damage. It is clear that oxidative stress-dependent angiogenesis is an important contributor to the progression of cancers and chronic diseases. Multifaceted pathways involved in oxidative stress-induced angiogenesis have yet to be investigated. Possible antioxidative strategies must be highly selective and may affect the production or scavenging of ROS or their metabolic products.
Acknowledgments
The authors thank Emelye Crehore for her assistance with manuscript proofreading.
This work was supported by a research grant from the National Institutes of Health, National Heart, Lung, and Blood Instutute (HL071625) (T.V.B.) and an American Heart Association Postdoctoral Fellowship (POST14570001) (Y.-W.K.).
Authorship
Contribution: Y.-W.K and T.V.B wrote the paper, and reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tatiana Byzova, Cleveland Clinic, 9500 Euclid Ave, NB-50, Cleveland, OH 44195; e-mail: byzovat@ccf.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal