Key Points
Exosomes in blood are proinflammatory and may contribute to transfusion-related immune modulation.
Exosomes act via antigen-presenting cells to potentiate T-cell survival and mitogen-induced proliferation.
Abstract
Extracellular vesicles (EVs) are small, double membrane vesicles derived from leukocytes, platelets, and cells of other tissues under physiological or pathological conditions. Generation of EVs in stored blood is thought to be associated with adverse effects and potentially immunosuppression in blood transfusion recipients. We measured the quantity and cells of origin for EVs isolated from stored red blood cell (RBC) units and tested whether they had any effects on T-cell–mediated immune responses. Mixing peripheral blood mononuclear cells (PBMCs) with EVs resulted in secretion of proinflammatory cytokines and chemokines and increased survival of unstimulated PBMCs. EVs augmented mitogen-induced CD4+ and CD8+ T-cell proliferation in an antigen-presenting cell (APC)-dependent manner. We demonstrated that EVs interacted primarily with monocytes and induced proinflammatory cytokine secretion. We also showed that the exosome fraction of EVs and not larger microvesicles was responsible for induction of TNF-α production by monocytes. Furthermore, blockade of CD40 or CD40L accessory molecules largely neutralized the EV augmentation of T-cell responses, implying a role for cell-cell interaction between T cells and EV-activated monocytes. Contrary to our hypothesis, the data demonstrate that EVs isolated from RBC units increase the potency of APCs and boost mitogen-driven T-cell proliferative responses.
Introduction
Extracellular vesicles (EVs) can be released from leukocytes, platelets, endothelial cells, and cells of other tissues under physiological or pathological conditions in response to activation, stress, necrosis, or apoptosis1-3 and can be found in body fluids.4,5 Three groups of EVs have been described according to their size and mechanism of generation: microvesicles are large cell membrane-derived particles in the range of 200 to 1200 nm.1,6 Exosomes, with an approximate size of 30 to 150 nm, are byproducts of exocytosis.5 Apoptotic bodies (50-500 nm) are the last group of EVs that are released from apoptotic cells.5
EVs may play immunosuppressive or immunostimulatory roles.7,8 It has been shown that C-phosphate-G (CPG)-stimulated B cells from HIV patients produce lower quantities of immunoglobulin G in the presence of EVs from the same patients.9 Platelet-derived EVs have been demonstrated to bias macrophages to an antiinflammatory response and secretion of transforming growth factor-β.6 However, exosomes bearing autoimmune antigens are immunostimulatory in a NOD mouse model of diabetes, leading to production of proinflammatory cytokines and proliferation of T cells.10
In this article we examine the role of EVs in potentially mediating an immune modulatory effect associated with blood transfusion. It is believed that transfusion of fresh blood may carry less risk of adverse reactions compared with old blood, attributed to a red blood cell (RBC) “storage lesion,” which has been described as physical and chemical changes of RBCs during the time of storage.11-13 Morphological changes to RBCs in stored packed-RBC units are accompanied by shedding and release of EVs from RBCs or from residual platelets and leukocytes in the bag.14-16 The overall balance of physical and chemical changes in stored blood may contribute to immunomodulation and potential adverse effects in patients who have received older blood, and EVs may be key mediators of immune modulation in transfusion recipients.7,12,13,17,18
EVs express different markers on their surface depending on their cell of origin, and they may contain RNA, DNA, and proteins.5,19 Increased generation of some EV subtypes has been associated with increased risk of specific diseases, and EVs may serve as valuable diagnostic biomarkers in the future.1,20-22 The cellular source of EVs and the immunomodulatory role of EVs generated during the storage of human RBC units are not well understood.7,23,24 Here, we tracked the quantity and cell of origin for EVs found in RBC units throughout the standard storage period. Furthermore, we hypothesized that RBC-EVs would suppress T-cell immune responses, and we tested whether EVs could modulate T-cell responses and whether antigen-presenting cells (APCs) participated in EV-driven modulation of the immune response.
Methods
Study samples
Six leukoreduced packed RBC units were received from Blood Centers of the Pacific. Peripheral blood mononuclear cells (PBMCs) from 6 donors were recovered from the leukoreduction chamber after platelet apheresis. PBMCs were purified and stored in liquid nitrogen. Supplemental Table 1 (available on the Blood Web site) provides more detail on packed cell preparation and apheresis technology. Written consent was obtained from the healthy blood donors in accordance with the Declaration of Helsinki, and the samples were de-identified. The study protocols were approved by the University of California, San Francisco Committees on Human and Animal Research.
Storage of packed RBC units and purification of EVs
Packed RBC units were split into 35-mL aliquots in replicate 180-mL transfer bags and stored at 4°C. EVs were isolated using differential centrifugation with an initial speed of 1500g to separate supernatant, followed by spinning the supernatant at 13 000g for preparation of platelet free supernatant. Three mL of platelet free supernatant was added to 32 mL phosphate-buffered saline and spun for 1 hour at 100 000g. EV pellets were resuspended in 1 mL RPMI and stored at −80°C. Fractionation of EVs to small and large EVs was performed with 0.22-μm centrifugal filters for 2 minutes at 750g (Millipore). Large EVs (microvesicles) were recovered from the top of the filters by washing, and the flow-through (small EVs) was termed exosomes.
Characterization of EVs
Fluorochrome-conjugated monoclonal antibodies were used to determine the origin of EVs (RBCs [CD235a-FITC], platelets [CD41a-PerCP/Cy5.5], lymphocytes [CD3-PerCP/Cy5.5, CD19-Alexa/700, and CD16-V450], monocytes [CD14-APC/Cy7], and endothelial cells [CD142-PE] [Biolegend]). One to 5 µL of titrated monoclonal antibodies was added to 100 µL of EVs. Expression of phosphatidylserine on EVs was examined by addition of 0.3 μg lactadherin-FITC to 100 μL EV preparations (Haematologic Technologies Inc.). Samples were incubated at 4°C for 20 minutes and resuspended in 400 µL phosphate-buffered saline. An LSR II flow cytometer (BD Biosciences) was used for data acquisition, and FlowJo 7.6.5 software (Tree Star) was used for data analysis. Forward scatter (FSC) and side scatter (SSC) parameters were set to log mode and low threshold and 0.2-, 1.0-, and 3.2-μm size control beads (Invitrogen) were used for FSC and SSC parameters. TruCount beads (BD Biosciences) were used to measure the absolute counts of EVs. Six-μm beads (Invitrogen) were coated with microvesicles or exosomes at 4°C for 18 hours on a shaker. Beads were washed and blocked with 5% bovine serum albumin at 4°C for 3 hours and immunostained with the exosome marker (CD63-APC, Biolegend) at 4°C for 30 min. Beads coated with bovine serum albumin alone were immunostained with the same antibody as the negative control.
Electron microscopy was performed on pelleted EVs. Pellets were fixed in 50 μL 2% glutaraldehyde-0.1 M phosphate solution for 24 hours at 4°C, then overlayed with 4% agar and cooled down to 24°C. Gels were rinsed 3 times with 0.1 M phosphate buffer for 5 minutes, fixed with 1% osmium tetroxide for 60 minutes, and rinsed with distilled water for 1 minute, followed by 35% ethanol for 30 minutes. Gels were kept in 1% paraphenelenediamine-70% ethanol at 4°C for 3 days and were rinsed in 1% paraphenelenediamine-95% ethanol for 15 minutes followed by washing 3 times with 100% ethanol for 15 minutes. Dehydration of the gels was performed using propylene oxide for 10 minutes, and samples were kept in propylene oxide/epoxy (1:1 wt%/wt%) overnight. Finally, gels were embedded in 100% epoxy resin and polymerized at 60°C for 48 hours. Samples were sectioned and stained with 2% uranyl acetate for 60 minutes and imaged with a JEOL JEM-1400 electron microscope.
Cytokine assays on PBMC supernatants
A multiplex MAG kit (Millipore) was used to measure the level of 39 cytokines, chemokines, and growth factors in culture supernatants. PBMCs at a concentration of 0.5 × 106/0.5 mL were cultured in 10% inactivated human serum in RPMI, containing 10 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid, 100 U/mL penicillin G, and 100 µg/mL streptomycin in the presence or absence of 100 µL EVs in a 5% CO2 incubator at 37°C for 24 hours. A Bio-Plex 200 instrument (Bio-Rad) was used for data acquisition, and data were plotted as a heat map by Aable software (Gigawiz). Interleukin (IL)-18 was measured by enzyme-linked immunosorbent assay (R&D Systems). Intracellular staining of EV-, exosome-, or microvesicle-stimulated PBMCs for TNF-α (-V421 labeled, Biolegend) was performed on 1 × 106/mL PBMCs after 6 hours. PMA- (50 ng/mL) and ionomycin- (250 ng/mL) stimulated PBMCs served as a positive control. Cells were stained with CD14-APC/Cy7, CD19-Alexa Fluor700, and CD3-PerCP/Cy5.5 antibodies (Biolegend) prior to permeabilization (BD Biosciences).
PBMC survival studies
Unstimulated or EV-stimulated (100 µL) PBMCs at a concentration of 0.5 × 106/0.5 mL media were cultured for 7 days. PBMCs were washed and stained with CD3-PerCp/Cy5.5, CD4-FITC, CD8-PE, CD19-Alexa/700, CD14-APC/Cy7, and CD56-PE/Cy7 antibodies (Biolegend), and data were acquired and analyzed as described above.
Proliferation assays
Proliferation of T cells was measured by staining PBMCs or purified T cells with carboxyfluorescein diacetate succinimidyl ester (CFSE) at a final concentration of 10 µM for 10 minutes. Five hundred thousand CSFE-stained PBMCs were cultured for 7 days in 0.5 mL media and were unstimulated or stimulated with 100 µL EV+/−phytohemagglutinin (PHA) (0.5 or 5 µg/mL, Sigma-Aldrich). PBMCs were harvested and stained for CD3-PerCp/Cy5.5, CD4-PE/Cy7, and CD8-PE cell surface markers (Biolegend), and data were acquired and analyzed as previously described.
Purification of T-cell and monocyte populations
Isolation of T lymphocytes or monocytes from PBMCs was performed by negative selection on PBMCs using the MagCellect human CD3+ T cell isolation kit (R&D Systems) or EasySep human monocyte enrichment kit (Stemcell Technologies), respectively.
Interaction of PBMC subpopulations with EVs
Purified monocytes or T cells were stained with PKH67 and EVs were stained with PKH26 cell membrane dyes (Sigma-Aldrich). Cells or EVs were washed twice with 10% fetal bovine serum in RPMI. Fifty thousand monocytes or T cells were cultured with 100 μL EV in a final volume of 250 μL for 3 or 24 hours. Cells were fixed with 2% paraformaldehyde and were analyzed at 24°C with a Leica 6000B fluorescence microscope (Leica microsystems) (×20/0.4D HCX PL FLUOTAR CORR objective lens). Images were captured with a Leica DFC365 FX camera, using Leica Application Suite software and were analyzed with ImageJ software (NIH). The same samples were analyzed by flow cytometry for quantification of the data.
CD40 and CD40L blocking antibodies
Functional grade blocking monoclonal antibodies against CD40 (Miltenyi Biotec) and CD40L (eBiosciences) were used for blockade of T-cell interaction with APCs in proliferation assays. Titration of antibodies was performed and the lowest dilution with maximal blocking was used (1.0 µg/mL).
Statistical analysis
Analyses of EV absolute count and composition, EV effects on PBMC survival, intracellular TNF-α level, and CD40 or CD40L blockade data were performed by Student paired t test. For analysis of cytokine data the Mann-Whitney test was used and false discovery rates were calculated for correction of multiple comparisons (q < 0.1 considered significant). For PBMC and T-cell proliferation experiments and for monocyte restoration assay with multiple conditions, analysis of variance was used with Tukey post-test. All analyses were performed by GraphPad Prism (version 5) and R (version 2.10.1) software.
Results
Characterization of EVs in stored RBC units
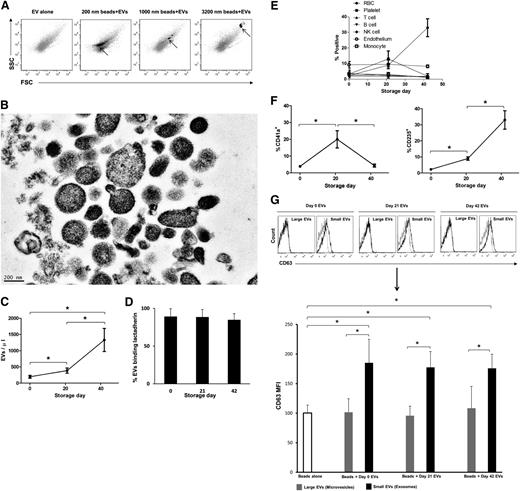
Flow cytometric analysis of EVs isolated from RBC units revealed a heterogeneous EV population; the majority was <1000 nm in diameter. The main population of EVs colocated with 200-nm microbeads on a FSC-SSC plot (Figure 1A). Electron microscopy confirmed the heterogeneity of EVs (∼50-600 nm), with many of them close to 200 nm in diameter (Figure 1B). Stored RBCs have been described to lose blebs of membrane during storage.25 Accordingly, EV counts rose by day 42 of storage (Figure 1C). Annexin-V reactivity of EVs was 15% to 35% and was dependent on the concentration of Ca++ (data not shown); however, 89% of EVs were reactive with lactadherin at 3 time points (Figure 1D). To measure the origin of the EVs, we used antibodies against cell surface antigens (Figure 1E; supplemental Figure 1). The mean percentage of platelet-derived CD41a+ EVs was 20% at day 21 and was at base line for other time points (Figure 1F, left). Protease activity was tested for a possible role of proteases in degradation of CD41+ EVs, and it was <1 ng/mL (supplemental Table 2). CD235a+ (RBC-derived) EVs increased from 2.3% at day 0 to 33.0% at day 42 (Figure 1F, right). The mean percentage of CD142+ (endothelial-derived) EVs was 7.5% on day 0 and varied little during the storage (Figure 1E). The percentage of lymphocyte and monocyte EVs (CD3, CD16, CD19, and CD14) was <5% at baseline and remained low throughout storage (Figure 1E). Contamination of packed cells with leukocytes was <0.1%, whereas 99.9% of cells were reactive with CD235a (supplemental Figure 2). Finally, day 0, 21, and 42 EVs were fractionated to <200 and >200 nm EVs. The small EVs were positive for the exosome marker26-28 CD63 and large EVs were not, demonstrating that small EVs were exosomes and large EVs were microvesicles (Figure 1G).
EV relative size, absolute count, and composition in stored RBC units. (A) Day 0 isolated EVs were subject to flow cytometry, and FSC-SSC data were acquired on log mode. The same settings were used for data acquisition using 0.2-, 1.0-, and 3.2-μm polystyrene beads, and the plots show the overlay of EVs (gray) and beads (black), representative of 3 independent experiments. (B) Electron micrograph of day 0 EVs prepared by positive staining, representative of 2 independent experiments (bar represents 200 nm). (C) Six RBC unit aliquots were tested by flow cytometry on day 0, 21, and 42 of storage at 4°C for absolute count of EVs (*P < .05). (D) EVs binding lactadherin-FITC (4 samples), measured by flow cytometry. (E) The following markers were used for characterization of EVs: RBC: CD235a; platelet: CD41a; T cell: CD3; B cell: CD19; natural killer cell: CD16; monocyte: CD14; and endothelial cell: CD142. (F) The increase of platelet- and RBC-derived EVs was significant at day 21. At least 50 000 EV events were acquired at each time point, and 4 independent experiments were performed for each condition (*P < .05). (G) A total of 6 EV samples (day 0, 21, 42) were fractionated into small or large particles using 0.22-μm centrifugal filters. Beads (6 µm) were coated with EVs and blocked using bovine serum albumin. The negative control was beads incubated with only bovine serum albumin and no EVs. Immunostaining was performed with the exosome marker CD63 on the small EV fraction (<200 nm, exosomes) and the large EV fraction (>200 nm, microvesicles). The negative control is shown as a gray line and EVs as a dark line (*P < .05).
EV relative size, absolute count, and composition in stored RBC units. (A) Day 0 isolated EVs were subject to flow cytometry, and FSC-SSC data were acquired on log mode. The same settings were used for data acquisition using 0.2-, 1.0-, and 3.2-μm polystyrene beads, and the plots show the overlay of EVs (gray) and beads (black), representative of 3 independent experiments. (B) Electron micrograph of day 0 EVs prepared by positive staining, representative of 2 independent experiments (bar represents 200 nm). (C) Six RBC unit aliquots were tested by flow cytometry on day 0, 21, and 42 of storage at 4°C for absolute count of EVs (*P < .05). (D) EVs binding lactadherin-FITC (4 samples), measured by flow cytometry. (E) The following markers were used for characterization of EVs: RBC: CD235a; platelet: CD41a; T cell: CD3; B cell: CD19; natural killer cell: CD16; monocyte: CD14; and endothelial cell: CD142. (F) The increase of platelet- and RBC-derived EVs was significant at day 21. At least 50 000 EV events were acquired at each time point, and 4 independent experiments were performed for each condition (*P < .05). (G) A total of 6 EV samples (day 0, 21, 42) were fractionated into small or large particles using 0.22-μm centrifugal filters. Beads (6 µm) were coated with EVs and blocked using bovine serum albumin. The negative control was beads incubated with only bovine serum albumin and no EVs. Immunostaining was performed with the exosome marker CD63 on the small EV fraction (<200 nm, exosomes) and the large EV fraction (>200 nm, microvesicles). The negative control is shown as a gray line and EVs as a dark line (*P < .05).
Cytokine profiles of stimulated PBMCs with EVs
It has been shown that incubation of macrophages with platelet-derived ectosomes biases them toward an antiinflammatory response.6 We hypothesized that EVs in RBC units would show a similar antiinflammatory effect, consistent with immune modulation that has been described in transfusion recipients.29,30 PBMCs were cultured unstimulated or stimulated with day 0, 21, and 42 EVs for 24 hours. Day 0 EVs induced significant upregulation of 27 cytokines (Figure 2A; P < .05, q < 0.1). Cytokine changes induced by day 21 and 42 EVs did not reach statistical significance, though the pattern of upregulation was the same. All the proinflammatory cytokines tested were upregulated, with the exception of the IL-12p40 subunit (Figure 2A). The antiinflammatory cytokines soluble CD40 ligand (sCD40L), IL-1Ra, and sIL-2Rα but not IL-5, IL-10, and IL-13 showed significant increases. Six of the 10 chemokines and 4 of the 7 growth factors in the panel were also significantly increased. We also found that IFN-γ, IL-17, and IL-9, but not IL-4, were significantly increased, implying upregulation of the T helper (Th)1, Th17, and Th9 but not Th2 pathways (Figure 2B). We noted that IL-1β was elevated to higher levels than many of the other cytokines and questioned whether the EVs might be activating the cells to produce IL-1β and IL-18 via the NLRP3 inflammasome pathway. To test this hypothesis, we measured IL-18; its level was not significantly increased (Figure 2C).
Cytokine and growth factor secretion by PBMCs stimulated with EVs. PBMCs were cultured unstimulated or stimulated with day 0, 21, and 42 EVs. Supernatants were collected at 24 hours and were tested using a multiplex cytokine assay for 39 cytokines and growth factors. Data are representative of PBMC culture supernatants from 3 healthy donors and 3 unstimulated and 6 EV-stimulated replicates for days 0 and 21 and 5 replicates for day 42 (*P < .05, q < 0.1, EV stimulated vs unstimulated condition). (A) Results for stimulated conditions were normalized to unstimulated data and log2 transformed to show log-fold increase after stimulation. (B) Cytokine secretion patterns associated with T-helper cell subsets. (C) Secretion pattern of IL-1β and IL-18. (D) Detailed presentation of T-cell growth factors. EGF, epidermal growth factor; FGF-2, fibroblast growth factor 2; Flt3L, FMS-related tyrosine kinase 3 ligand; Gro, growth regulated protein (CXCL3); MCP-1, monocyte chemoattractant protein 1 (CCL2); MCP-3, monocyte chemoattractant protein 3 (CCL7); MDC, macrophage-derived chemokine (CCL22); MIP-1α, macrophage inflammatory protein 1α (CCL3); MIP-1β, macrophage inflammatory protein 1β (CCL4); VEGF, vascular endothelial growth factor.
Cytokine and growth factor secretion by PBMCs stimulated with EVs. PBMCs were cultured unstimulated or stimulated with day 0, 21, and 42 EVs. Supernatants were collected at 24 hours and were tested using a multiplex cytokine assay for 39 cytokines and growth factors. Data are representative of PBMC culture supernatants from 3 healthy donors and 3 unstimulated and 6 EV-stimulated replicates for days 0 and 21 and 5 replicates for day 42 (*P < .05, q < 0.1, EV stimulated vs unstimulated condition). (A) Results for stimulated conditions were normalized to unstimulated data and log2 transformed to show log-fold increase after stimulation. (B) Cytokine secretion patterns associated with T-helper cell subsets. (C) Secretion pattern of IL-1β and IL-18. (D) Detailed presentation of T-cell growth factors. EGF, epidermal growth factor; FGF-2, fibroblast growth factor 2; Flt3L, FMS-related tyrosine kinase 3 ligand; Gro, growth regulated protein (CXCL3); MCP-1, monocyte chemoattractant protein 1 (CCL2); MCP-3, monocyte chemoattractant protein 3 (CCL7); MDC, macrophage-derived chemokine (CCL22); MIP-1α, macrophage inflammatory protein 1α (CCL3); MIP-1β, macrophage inflammatory protein 1β (CCL4); VEGF, vascular endothelial growth factor.
EVs promote PBMC survival
Elevated levels of IL-2, IL-7, and IL-15 growth factors in EV-stimulated PBMCs (Figure 2D) prompted us to measure the absolute count of unstimulated and EV-stimulated PBMCs 7 days post-culture. The average absolute count of unstimulated PBMCs decreased from 1 × 106/mL to 0.7 × 106/mL, whereas the average absolute count of EV-stimulated PBMCs was maintained at baseline levels (Figure 3A). EVs enhanced the survival of T-cell, B-cell, monocyte, and natural killer cell populations to a similar degree, consistent with induction of a broad array of growth factors (Figure 3B). It was possible EVs induced increased homeostatic cell turnover, and this was tested by staining PBMCs with CFSE prior to EV exposure; however, we did not observe T-cell proliferation in unstimulated or EV-exposed PBMCs (Figure 4A).
EV effects on PBMC survival. PBMCs were cultured for 7 days with or without addition of day 0 EVs. Cell counts were performed at days 0 and 7, and at harvest cells were stained for CD3, CD4, CD8, CD19, CD14, and CD56 expression. Five PBMC preparations and 5 independent EV isolates were used for this experiment (**P < .01). (A) Absolute counts of PBMCs at day 7 relative to baseline count of 1 × 106/mL. (B) Percentage of cells expressing markers of T cells, B cells, monocytes, and natural killer cells at day 7.
EV effects on PBMC survival. PBMCs were cultured for 7 days with or without addition of day 0 EVs. Cell counts were performed at days 0 and 7, and at harvest cells were stained for CD3, CD4, CD8, CD19, CD14, and CD56 expression. Five PBMC preparations and 5 independent EV isolates were used for this experiment (**P < .01). (A) Absolute counts of PBMCs at day 7 relative to baseline count of 1 × 106/mL. (B) Percentage of cells expressing markers of T cells, B cells, monocytes, and natural killer cells at day 7.
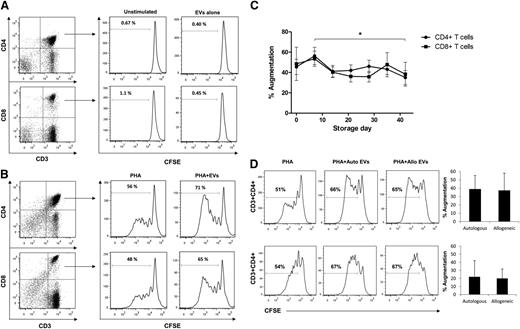
EV augmentation of T lymphocyte proliferation. PBMCs were stained with CFSE and cultured for 7 days, stained with CD3, CD4, and CD8 monoclonal antibodies, and proliferation was quantified as the percentage of CFSElow cells on CD3+CD4+ or CD3+CD8+ T cells. (A) T-cell proliferation was not detectable when PBMCs were stimulated with EVs alone. (B) Proliferation of CD3+CD4+ and CD3+CD8+ T cells stimulated with PHA or PHA plus day 0 EV. (C) T-cell proliferation changes induced by EVs derived from RBCs stored up to 42 days. Proliferation of CD3+CD4+ and CD3+CD8+ T cells is shown as percent augmentation relative to PHA alone stimulation. Six independent experiments were performed at each time point. Six PBMC preparations and 6 independent EV isolates were used for this experiment (*P < .05, day 7 vs day 42 EVs). (D) Proliferation of CD3+CD4+ and CD3+CD8+ T cells in response to day 0 autologous or allogeneic EVs did not show a significant difference. A total of 4 independent autologous and allogeneic experiments were performed.
EV augmentation of T lymphocyte proliferation. PBMCs were stained with CFSE and cultured for 7 days, stained with CD3, CD4, and CD8 monoclonal antibodies, and proliferation was quantified as the percentage of CFSElow cells on CD3+CD4+ or CD3+CD8+ T cells. (A) T-cell proliferation was not detectable when PBMCs were stimulated with EVs alone. (B) Proliferation of CD3+CD4+ and CD3+CD8+ T cells stimulated with PHA or PHA plus day 0 EV. (C) T-cell proliferation changes induced by EVs derived from RBCs stored up to 42 days. Proliferation of CD3+CD4+ and CD3+CD8+ T cells is shown as percent augmentation relative to PHA alone stimulation. Six independent experiments were performed at each time point. Six PBMC preparations and 6 independent EV isolates were used for this experiment (*P < .05, day 7 vs day 42 EVs). (D) Proliferation of CD3+CD4+ and CD3+CD8+ T cells in response to day 0 autologous or allogeneic EVs did not show a significant difference. A total of 4 independent autologous and allogeneic experiments were performed.
EV-induced augmentation of T-cell responses
We next tested whether EVs could modulate mitogen-driven T-cell immune responses. Indeed, EVs significantly augmented proliferation of CD4+ and CD8+ T cells in response to PHA stimulation (Figure 4B). We also tested whether EV-driven immune modulation would change if the EVs were derived from older RBCs. Whereas PHA+EV cultures showed significantly enhanced CD4+ and CD8+ T-cell proliferation compared with PHA alone at each time point, levels of EV-induced proliferation augmentation did not change with RBC unit storage time (with the only difference seen for day 7 vs day 42 for CD4+ T cells) (Figure 4C). EV-induced proliferation augmentation was the same when autologous or allogeneic EVs were used, implying that the EV stimulatory effect was not driven by alloreactivity (Figure 4D).
APC help is necessary and sufficient for EV-induced augmentation of T-cell proliferation
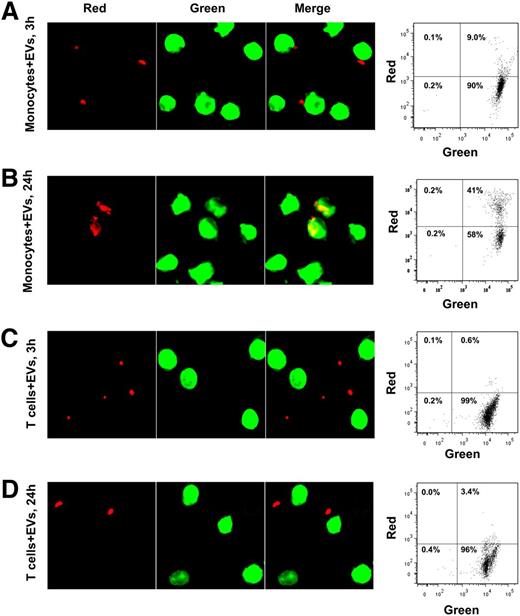
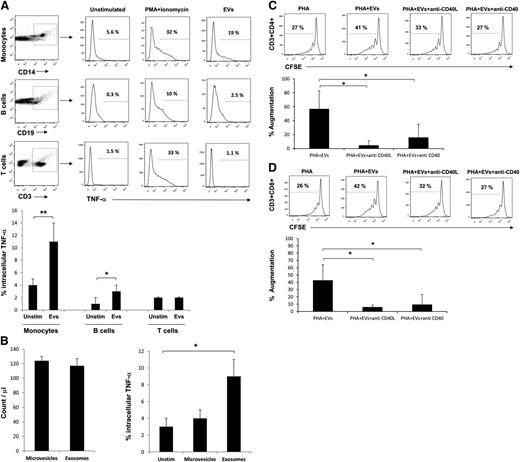
We next were interested to know if EVs augmented T-cell proliferation via a direct effect on T cells or through an effect on APCs. Stimulation of purified T cells with low concentration PHA+/−EV did not induce proliferation of T-cell subpopulations (Figure 5A top panels). Whereas purified T cells responded to high concentrations of PHA, the addition of EVs did not augment their proliferative response (Figure 5A, lower panels). Addition of purified monocytes to purified T cells recapitulated the EV-induced augmentation of T-cell proliferation, demonstrating that APCs are needed to mediate the effect (Figure 5B). Finally, we asked if APCs or T cells preincubated with EVs and then washed could still augment T-cell proliferation (Figure 5C). When monocytes plus EVs were incubated for 1 hour and then washed, addition of PHA and T cells augmented proliferation in CD4+ and CD8+ T cells, whereas preincubation of T cells plus EVs for 1 hour followed by washing and addition of PHA and monocytes did not lead to augmented proliferation in CD4+ and CD8+ T cells (Figure 5D-E). To show that EVs directly interact with monocytes and not T cells, purified monocytes or T cells were stained with a green membrane dye. EVs were stained with a red membrane dye, and monocytes or T cells were incubated with them. Interaction and uptake of EVs by monocytes were observed by fluorescence microscopy and confirmed by flow cytometry (Figure 6A-B), but T cells did not appreciably interact with EVs (Figure 6C-D). We hypothesized that because EVs interacted primarily with monocytes, these cells would be the primary producers of proinflammatory cytokines in PBMCs. As TNF-α was robustly elevated after EV stimulation of PBMCs (Figure 2A), we performed intracellular staining for this cytokine in EV-stimulated PBMCs. Monocytes in fact were the main source of TNF-α, with some production seen in B cells (Figure 7A). We next explored whether exosomes were the biologically active fraction via size separation. Particles <200 nm (exosomes) stimulated monocytes to produce TNF-α, whereas equal numbers of larger microvesicles did not (Figure 7B). Finally, we showed that blockade of CD40 or CD40L costimulatory molecules in PBMC cultures prevented EV-induced T-cell proliferation (Figure 7C-D). These results demonstrate that exosomes alter monocytes to augment T-cell proliferation.
Mechanism of EV-induced immune augmentation. T cells were stained with CFSE and cultured alone or after restoration of monocytes for 7 days in PHA or PHA+EV conditions. Cells were stained for CD3, CD4, and CD8 cell surface markers. (A) Stimulation of purified T cells with low (0.5 µg/mL) or high (5.0 µg/mL) PHA concentration. (B) Addition of purified monocytes to purified T cells prior to addition of low concentration of PHA+/−EVs. (C) Schematic experimental design for testing the effect of EVs on purified T cells or purified monocytes. (D-E) Incubation of EVs with purified CD4+ (D) or CD8+ (E) T cells or purified monocytes for 1 hour followed by washing and mixing with purified monocytes or T cells, respectively, and as outlined in diagram (C). Data are representative of 3 independent experiments (*P < .05, **P < .01).
Mechanism of EV-induced immune augmentation. T cells were stained with CFSE and cultured alone or after restoration of monocytes for 7 days in PHA or PHA+EV conditions. Cells were stained for CD3, CD4, and CD8 cell surface markers. (A) Stimulation of purified T cells with low (0.5 µg/mL) or high (5.0 µg/mL) PHA concentration. (B) Addition of purified monocytes to purified T cells prior to addition of low concentration of PHA+/−EVs. (C) Schematic experimental design for testing the effect of EVs on purified T cells or purified monocytes. (D-E) Incubation of EVs with purified CD4+ (D) or CD8+ (E) T cells or purified monocytes for 1 hour followed by washing and mixing with purified monocytes or T cells, respectively, and as outlined in diagram (C). Data are representative of 3 independent experiments (*P < .05, **P < .01).
Interaction of PBMC subpopulations with EVs. Monocytes and T cells were purified from PBMCs and stained with PKH67 green membrane dye. EVs were stained with PKH26 red membrane dye. EVs were added to monocytes or T cells and incubated in a 5% CO2 incubator for 3 or 24 hours. Cells were fixed with 2% PFA and subject to fluorescence microscopy or flow cytometry. Data are representative of 3 independent experiments. (A) Monocytes 3 hours after incubation with EVs. (B) Monocytes 24 hours after incubation with EVs. (C) T cells 3 hours after incubation with EVs. (D) T cells 24 hours after incubation with EVs.
Interaction of PBMC subpopulations with EVs. Monocytes and T cells were purified from PBMCs and stained with PKH67 green membrane dye. EVs were stained with PKH26 red membrane dye. EVs were added to monocytes or T cells and incubated in a 5% CO2 incubator for 3 or 24 hours. Cells were fixed with 2% PFA and subject to fluorescence microscopy or flow cytometry. Data are representative of 3 independent experiments. (A) Monocytes 3 hours after incubation with EVs. (B) Monocytes 24 hours after incubation with EVs. (C) T cells 3 hours after incubation with EVs. (D) T cells 24 hours after incubation with EVs.
TNF-α production indicates monocytes as primary interacting cells with EVs, and blockade of CD40 or CD40L accessory molecules decreases EV-induced T-cell augmentation response. (A) Intracellular staining of PBMCs for TNF-α and cell surface staining for monocytes (**P < .01), B cells (*P < .05), and T cells. Data are representative of 4 independent experiments, 6 hours post-stimulation of PBMCs with EVs. (B) Left: The absolute counts of exosomes and microvesicles were measured by TruCount beads (n = 3). Right: Three PBMC sets were stimulated with exosome or microvesicle fractions derived from a unique EV sample, and intracellular TNF-α was measured after 6 hours. Stimulation with exosomes but not microvesicles led to production of TNF-α in monocytes (*P < .05). (C-D) PBMCs were stained with CFSE. Anti-CD40 or anti-CD40L blocking antibodies were added to the culture in the presence of PHA+EV. Cells were harvested at day 7 and stained for CD3, CD4, and CD8 cell surface markers. Addition of blocking antibodies reduced augmentation of CD4+ and CD8+ T cells (*P < .05). Data are representative of 4 independent experiments.
TNF-α production indicates monocytes as primary interacting cells with EVs, and blockade of CD40 or CD40L accessory molecules decreases EV-induced T-cell augmentation response. (A) Intracellular staining of PBMCs for TNF-α and cell surface staining for monocytes (**P < .01), B cells (*P < .05), and T cells. Data are representative of 4 independent experiments, 6 hours post-stimulation of PBMCs with EVs. (B) Left: The absolute counts of exosomes and microvesicles were measured by TruCount beads (n = 3). Right: Three PBMC sets were stimulated with exosome or microvesicle fractions derived from a unique EV sample, and intracellular TNF-α was measured after 6 hours. Stimulation with exosomes but not microvesicles led to production of TNF-α in monocytes (*P < .05). (C-D) PBMCs were stained with CFSE. Anti-CD40 or anti-CD40L blocking antibodies were added to the culture in the presence of PHA+EV. Cells were harvested at day 7 and stained for CD3, CD4, and CD8 cell surface markers. Addition of blocking antibodies reduced augmentation of CD4+ and CD8+ T cells (*P < .05). Data are representative of 4 independent experiments.
Discussion
Here we have shown that the absolute count of EVs in leuko-reduced RBC units increased with storage time, mainly due to the increase of RBC- and platelet-derived EVs. Endothelial-derived EVs were present from day 0 in the stored blood, and their level did not change during the storage time. EVs induced production of predominantly proinflammatory cytokines and chemokines in PBMCs. Exosomes were the bioactive fraction of EVs that induced TNF-α secretion in monocytes. The net effects of exposure to EVs were improved survival of unstimulated cells and augmentation of mitogen-driven T-cell responses. EVs did not act directly on T cells, but rather through APCs and their subsequent interaction with T cells, at least in part through CD40/CD40L. These results shed new light on how transfusion might contribute to transfusion-related immune modulation and how EVs can interact with APCs to influence T-cell responses.
Leukoreduction of blood with in-line filters reduces the number of leukocytes to <5 million/bag, a 99.9% reduction.8,31 As expected, we did not observe notable EV percent increases for leukocyte markers in our experiments. RBC units are more prone to platelet and EV contamination than leukocyte contamination. It has been shown that only 99% of platelets and 72% of platelet-derived EVs are eliminated after leukofiltration.8 In the current study, endothelial-derived EVs were detectable in leukoreduced packed RBCs, and their level did not change with storage, in line with the above finding that leukofiltration cannot efficiently remove all EVs. Platelet-derived EVs peaked at day 21, consistent with the increase in platelet-derived EVs recently shown during refrigerated, short-term storage of whole blood.32 The proportion of platelet-derived EVs returned to baseline at day 42, potentially as a result of their faster degradation during storage relative to RBC-derived EVs. It has been shown that procaspase 3, caspase 8, and calpain in RBCs are involved in RBC senescence33-35 ; however, we could not detect the activity of proteases in stored blood supernatant, and degradation of platelet-derived EVs did not appear to be the result of protease activity. Flow cytometric and electron microscopic analysis of the EVs showed that our EV preparations were a mixture of microvesicles and exosomes.36
The function of immune cells in response to EVs is controversial, and EVs may skew the immune system toward suppression or stimulation.37-39 It has been demonstrated that stimulation of macrophages with platelet-derived ectosomes drives them to an antiinflammatory profile.6 Additionally, tumor-derived EVs are immunosuppressive and can inhibit the expansion of IL-2–induced CTL and natural killer cells, but not regulatory T cells, in PBMC cultures.40 Tumor-derived EVs can also induce myeloid-derived suppressor cells.41,42 Although several studies support an immune suppressive role for EVs, these are balanced by those suggesting a proinflammatory effect. Stimulation of monocytes with apoptotic platelet EVs leads to their differentiation into resident M2 macrophages that produced proinflammatory cytokines.43 In addition, EVs derived from LPS-stimulated monocytes or dendritic cells (DCs) activate CD4+ T cells.44 Our data are consistent with the latter studies in that EVs in RBC units were proinflammatory.
In addition to biasing the immune system, EVs can deliver antigen-specific signals. Exosomes from the Min6 mouse insulinoma cell-line have been shown to contain antigens that trigger autoimmunity in NOD mice.10 In preliminary mouse experiments, we did not detect an in vivo immune effect in BALB/c mice after injection of EVs purified from leukoreduced RBC units from C57BL/6 mice (supplemental Figure 3), potentially due to limitations of the mouse model in recapitulating the human system. Of note, in our human experiments, the immune effect did not appear to be antigen specific, as EV stimulatory capacity did not differ when EVs were derived from autologous vs allogeneic cells. Our study indicated that EVs from all time points of the storage of blood were capable of inducing significant T-cell proliferation augmentation. The ratio of RBC-EVs to platelet-EVs increased with storage time but did not affect the potency of the immune modulatory effects of the EVs, implying that the effects we measured were likely not limited to EVs expressing RBC or platelet antigens and could be from EVs derived from endothelial cells45 or other sources we did not measure. Our cytokine data showed a marginally greater up-regulation of proinflammatory cytokines by day 0 EVs compared with day 21 or 42 EVs (Figure 2A). We also found that monocytes are the main source of proinflammatory cytokine TNF-α in response to EVs, and the exosome fraction induced TNF-α production. These results imply that the EV component of fresh blood may have stronger immune modulatory potential, which would need to be confirmed with in vivo studies. This would have potentially important implications for understanding the effects of blood storage age on clinical outcomes.46
It has recently been shown that tumor-derived exosomes can activate myeloid-derived suppressor cells via the toll like receptor pathway.41,42 Analysis of EVs generated by mycobacteria-infected macrophages revealed that they colocalized with the endocytic recycling marker Rab11a but not with markers of early or late endosomes or lysosomes.47 In our multiplex test of cytokines up-regulated by EVs, IL-1β showed a relatively high fold-change, prompting us to measure IL-18 to test if this other effector cytokine of the NLRP3 inflammasome pathway would be coordinately up-regulated. The lack of EV-induced IL-18 secretion suggests that IL-1β and IL-18 may have been upregulated downstream of different PAMPs.
As uptake of EVs by monocytes has been shown by other investigators,47,48 we next hypothesized that EV-induced T-cell proliferation is APC-dependent and requires cell-cell contact. It has been shown that exosomes from acute HIV-1 patients exert an inhibitory effect on CD4+ T cells in a DC-dependent manner.49 Incubation of plasmacytoid DCs with EVs derived from TNF-α–activated endothelial cells led to stimulation and maturation of DCs, release of IL-6 and IL-8, and proliferation of naïve CD4+ T cells.45 Our data show that the presence of monocytes is necessary and sufficient for EV-induced T-cell response augmentation, which is consistent with the findings of several studies.40,45,47,49 Our data also suggest that cell-cell contact is necessary, as EVs interacted directly with monocytes and not T cells, and blockade of CD40 or CD40L prevented T-cell proliferation augmentation.50,51
The association of EV subtypes with disease has been a focus of some clinical trials, and EV characterization may lead to the introduction of new biomarkers for diagnostic purposes.21,22 The role of EVs in immunomodulation may also lead to the development of new treatment protocols in clinical settings. For instance, it has been shown that EVs from HIV patients9,49 or those derived from tumor cells40 have suppressive effects, and one can speculate that neutralization or inhibition of their generation might improve patient outcomes. On the other hand, the stimulatory role of some EV subtypes may be useful for inhibition of tumor growth and treatment of cancer.52 As we better define the characteristics and mechanism of action of EVs in transfusion products, we will have a novel parameter to follow in developing better storage methods to limit the putative blood storage lesion. Additionally, appreciation of any detrimental role of EVs would spur development of procedures to remove these potentially hazardous materials after storage and before transfusion of blood to patients.
Here, we have shown that EVs found in stored RBC units were devoid of leukocyte markers we measured, though these EVs were immunoreactive with endothelial, RBC, and platelet markers. The exosome fraction was capable of stimulating PBMCs in vitro and provoking proinflammatory cytokine response. EVs augmented mitogen-driven T-cell proliferation in PBMC cultures in an APC- and cell contact-dependent manner. Our data suggest that EVs are potential modifiers of T-cell responses in addition to their reported roles in innate immunity and B-cell responses.6,9,53
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dale Hirschkorn from Blood Systems Research Institute for his help with flow cytometer instrument settings and Brenda Campos from Blood Centers of the Pacific for preparing the leukoreduced packed RBC units. We thank Larry Ackerman from Microscopy Core of Diabetes and Endocrinology Research Center at University of California, San Francisco for processing our EV samples and data acquisition with electron microscopy. We also thank Jacqueline Law for her help with proliferation assays.
This study was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute grant HL095470.
Authorship
Contribution: A.D. designed the experiments, purified the EVs, harvested mice spleens, performed the human and mouse experiments, analyzed and interpreted the data, and wrote the manuscript; H.C.I. purified the EVs and isolated mice splenocytes; R.P.J. coordinated the mouse experiments, harvested mouse blood, and helped with mouse injections; S.W. performed statistical analyses of the data; X.D. assisted with statistical analysis of cytokine data; M.O.M. performed the injection of EVs to mice; J.W.H. performed cytokine assays; and P.J.N. designed the experiments, interpreted the data, and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philip J. Norris, 270 Masonic Ave, San Francisco, CA 94118; e-mail: pnorris@bloodsystems.org.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal