Abstract
A 35-year-old woman with recurrent severe placenta-mediated pregnancy complications in her 2 pregnancies asks: Will low-molecular-weight heparin help prevent recurrent placenta-mediated pregnancy complications in my next pregnancy? We performed a meta-analysis of randomized controlled trials (RCTs) comparing low-molecular-weight heparin (LMWH) vs no LMWH for the prevention of recurrent placenta-mediated pregnancy complications. We identified six RCTs that included a total of 848 pregnant women with prior placenta-mediated pregnancy complications. The primary outcome was a composite of pre-eclampsia (PE), birth of a small-for-gestational-age (SGA) newborn (<10th percentile), placental abruption, or pregnancy loss >20 weeks. Overall, 67 (18.7%) of 358 of women being given prophylactic LMWH had recurrent severe placenta-mediated pregnancy complications compared with 127 (42.9%) of 296 women with no LMWH (relative risk reduction, 0.52; 95% CI, 0.32 to 0.86; P = .01; I2, 69%, indicating moderate heterogeneity). We identified similar relative risk reductions with LMWH for individual outcomes, including any PE, severe PE, SGA <10th percentile, SGA <5th percentile, preterm delivery <37 weeks, and preterm delivery <34 weeks with minimal heterogeneity. LMWH may be a promising therapy for recurrent, especially severe, placenta-mediated pregnancy complications, but further research is required.
Introduction
A successful pregnancy requires the development of adequate placental circulation. Placenta-mediated pregnancy complications, including pre-eclampsia (PE), late pregnancy loss, placental abruption, and small-for-gestational-age (SGA) newborn, are distressing and often devastating pregnancy outcomes for women, their families, and society. Hypothesized to be a result of placental insufficiency, these complications are common, affecting more than 1 in 6 pregnancies.1 It has been postulated that placental vascular thrombosis and abnormal placentation are at least partly responsible for the placenta-mediated pregnancy complications.2-4 It has been postulated that anticoagulants might prevent placental thrombosis and in turn might prevent placenta-mediated pregnancy complications.
The risk of recurrent placenta-mediated pregnancy complications in subsequent pregnancies is substantial. For example, women with prior severe PE will have a 25% to 65% risk of recurrent PE, 3% risk of placental abruption, and 10% risk of SGA (<10th percentile).5,6 These complications may be multiple (eg, both PE and SGA) and are not isolated to the placenta-mediated complication experienced in a prior pregnancy.5,7 There are no highly effective preventative strategies in subsequent pregnancies, with only aspirin offering very small relative risk (RR) reductions in patients with prior PE.8 Recent randomized controlled trials (RCTs) conducted to determine whether low-molecular-weight heparin (LMWH) can prevent recurrent placenta-mediated pregnancy complications suggest an important treatment effect,9-14 but this finding has not been universal.13
Given the importance of preventing recurrent placenta-mediated pregnancy complications, limited current preventative options, and these early promising trials, we sought to determine a summary effect from randomized trials comparing prophylactic LMWH with no LMWH in pregnant women with prior placenta-mediated pregnancy complications.
Methods
We conducted a systematic review and meta-analysis following a systematic review protocol developed a priori in February 2011 and available on request. A systematic literature search was conducted to identify potential studies by using MEDLINE (1950 to September week 4 2012), Embase (1980 to 2012 week 12), the Cochrane Register of Controlled Trials (2nd quarter 2012), and OVID HealthSTAR (1999 to February 2012) using the OVID interface. Publications were also sought through a hand search of potentially relevant journals. The systematic search strategy is documented in the supplemental Data, available on the Blood Web site. The search was restricted to humans. There were no restrictions on language, publication year, or type of publication. References of included studies and narrative reviews were searched for potential studies. Publications were also sought through a hand search of conference proceedings of the American Society of Hematology Annual Meeting (1999 to 2011), International Society of Thrombosis and Hemostasis (1999 to 2011), and Women’s Health Issues in Thrombosis and Hemostasis (2009 to 2011). We also contacted all study authors of relevant abstracts and experts in the field to identify additional studies. The search was updated in May 2013 and no additional relevant articles were identified. Abstracts were reviewed by two reviewers (M.C. and M.A.R.) who independently and in duplicate determined whether the publication satisfied the following eligibility criteria: (1) the study population included currently pregnant women with prior pregnancies complicated by one or more of the following: PE, placental abruption, SGA newborn (<10th percentile), or pregnancy loss >12 weeks (one or more); (2) RCTs comparing participants who received LMWH with/without aspirin (ASA) compared with no LMWH control with/without ASA; (3) the primary outcome of interest for this meta-analysis was a composite of any PE, placental abruption, SGA newborn (<10th percentile), or pregnancy loss >20 weeks in the study pregnancy. Patients with more than one composite outcome were counted once. A composite outcome was selected because of the frequent overlap of these complications (eg, pregnancies complicated by PE are more likely to have placental abruption and/or birth of an SGA child or late pregnancy loss). Secondary outcomes included a composite of severe (as defined by the authors) or early onset (<34 weeks) PE, major abruption (as defined by the authors), SGA child (<5th percentile), or pregnancy loss >20 weeks and the following individual outcomes: any PE (as defined by the authors), severe (as defined by the authors) or early onset PE (<34 weeks), SGA <5th percentile, SGA <10th percentile, pregnancy loss >20 weeks, placental abruption, delivery <34 weeks, and delivery <37 weeks.
Data extraction was conducted independently and in duplicate by using piloted forms (M.A.R. and M.C.). Disagreements were resolved by consensus. We contacted the authors for data clarifications (5 authors provided responses to queries and one did not). Data extraction included the number of participants, study-level inclusion and exclusion criteria, intervention and control details (drug, dose), ASA co-intervention, patient characteristics, including prior history of each of the placenta-mediated pregnancy complications, and outcome data (primary composite, secondary composite, and individual secondary outcomes listed above). We assumed that patients reported to have SGA <5th percentile had SGA <10th percentile.
Quality assessment was conducted for all eligible publications by using the risk of assessment bias tool from the Cochrane Handbook for Randomized Trials.15
Data synthesis was conducted with StatsDirect Statistical Software Version 2.7.8 (Cheshire, United Kingdom). We examined RR and 95% confidence intervals (CIs) around RRs by using random effects models. Analyses were done with intention to treat. We explored heterogeneity and consistency of effects across studies with Higgins I2. Higgins suggests categorization of I2 into low (<25%), moderate (25% to 75%), and high (>75%) heterogeneity.15
Results
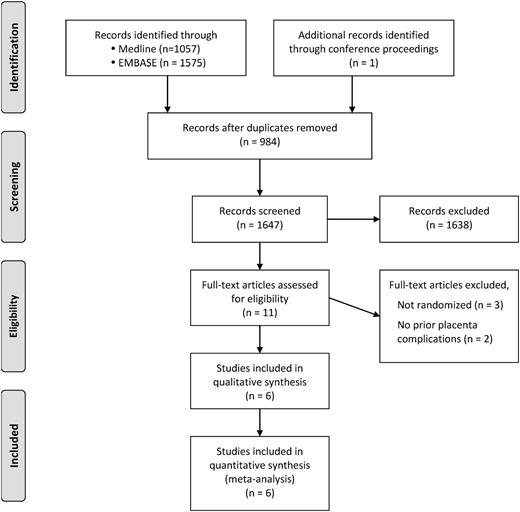
Our search strategy (supplemental Data) identified 1647 potential publications for review, from which we identified six RCTs that met our eligibility criteria (Figure 1).
The details of the included studies are provided in Table 1, and summary characteristics of included participants are provided in Table 2. The majority of randomized participants were recruited in single-center studies, with more than 40% recruited from one center (Nimes, France). Most of the participants had prior PE (70%), with the majority of these (70%) having had severe or early-onset PE. One quarter of participants had an identified thrombophilia. Some studies included only thrombophilic participants,12 some permitted inclusion of thrombophilic participants,9,11,13 and some excluded participants with known thrombophilia.10,14 In all studies, the intervention was a standard prophylactic dose of a marketed LMWH. Although the primary outcome variable was available for all study participants, some secondary outcomes could not be extracted from the publication nor were they provided by the authors (unavailable after requested). The quality of each of the included studies is reviewed in Table 3. There were no double-blind studies. All of the studies had adequate random sequence generation; most studies had adequate allocation concealment (5/6), half the studies reported blind independent adjudicated outcomes (3/6), and two of the studies reported a priori clinical trial registration12,13 to limit selective reporting.
Characteristics of studies included in the meta-analysis
| Reference . | Year . | Country . | Center . | No. of participants . | Previous outcome . | Intervention/control . | Primary outcome . |
|---|---|---|---|---|---|---|---|
| 12 | 2012 | Multi-national | 139 | Prior early onset PE (n = 107) and/or SGA <10th percentile (n = 94) | Dalteparin 5000 IU + ASA vs ASA | PE prior to 34 wk GA | |
| 13 | 2012 | Italy | Multi-center | 135 | Prior PE (n = 52), prior loss >15 weeks (n = 49), prior SGA <10th percentile (n = 28), or prior abruption (n = 5) | Nadroparin 3800 IU vs no nadroparin | PE, loss >15 wk GA, SGA <10th percentile, and/or abruption |
| 9 | 2011 | France | Single center | 224 | Prior severe PE (n = 224) | Enoxaparin 4000 IU + ASA vs ASA | PE, SB, abruption, SGA <5th percentile |
| 11 | 2010 | France | Single center | 160 | Prior abruption (n = 160; 70 with PE) | Enoxaparin 4000 IU ± ASA vs ± ASA | PE, SB, abruption, SGA <5th percentile |
| 10 | 2009 | Canada | Multi-center | 116 | Prior early PE (n = 60) | Dalteparin 5000 IU ± ASA vs ± ASA | PE, SB, abruption, SGA <5th percentile |
| Prior abruption (n = 16) | |||||||
| Prior SGA <5th percentile (n = 21) | |||||||
| Loss >12 wk (n = 17) | |||||||
| 14 | 2005 | Italy | Single center | 80 | Prior PE with ACE DD (n = 80) | Dalteparin 5000 IU vs no dalteparin | PE, SGA <10th percentile |
| Reference . | Year . | Country . | Center . | No. of participants . | Previous outcome . | Intervention/control . | Primary outcome . |
|---|---|---|---|---|---|---|---|
| 12 | 2012 | Multi-national | 139 | Prior early onset PE (n = 107) and/or SGA <10th percentile (n = 94) | Dalteparin 5000 IU + ASA vs ASA | PE prior to 34 wk GA | |
| 13 | 2012 | Italy | Multi-center | 135 | Prior PE (n = 52), prior loss >15 weeks (n = 49), prior SGA <10th percentile (n = 28), or prior abruption (n = 5) | Nadroparin 3800 IU vs no nadroparin | PE, loss >15 wk GA, SGA <10th percentile, and/or abruption |
| 9 | 2011 | France | Single center | 224 | Prior severe PE (n = 224) | Enoxaparin 4000 IU + ASA vs ASA | PE, SB, abruption, SGA <5th percentile |
| 11 | 2010 | France | Single center | 160 | Prior abruption (n = 160; 70 with PE) | Enoxaparin 4000 IU ± ASA vs ± ASA | PE, SB, abruption, SGA <5th percentile |
| 10 | 2009 | Canada | Multi-center | 116 | Prior early PE (n = 60) | Dalteparin 5000 IU ± ASA vs ± ASA | PE, SB, abruption, SGA <5th percentile |
| Prior abruption (n = 16) | |||||||
| Prior SGA <5th percentile (n = 21) | |||||||
| Loss >12 wk (n = 17) | |||||||
| 14 | 2005 | Italy | Single center | 80 | Prior PE with ACE DD (n = 80) | Dalteparin 5000 IU vs no dalteparin | PE, SGA <10th percentile |
ACE DD, angiotensin converting enzyme deletion/deletion genotype; GA, gestational age; SB, stillbirth.
Summary characteristics of participants in the studies included in the meta-analysis
| . | LMWH (n = 425) . | No LMWH (n = 423) . | Combined (n = 848) . | |
|---|---|---|---|---|
| n/N . | n/N . | n/N . | % . | |
| Thrombophilia | 106/425 | 107/423 | 213/848 | 25 |
| Prior PE | 296/425 | 293/423 | 593/848 | 70 |
| Prior severe PE | 208/304 | 208/304 | 416/608 | 68 |
| Prior SGA <10th percentile | 76/192 | 67/192 | 143/384 | 37 |
| Prior abruption | 91/192 | 90/203 | 181/405 | 45 |
| Prior loss >12 wk | 34/122 | 32/123 | 66/245 | 27 |
| Concomitant ASA use | 178/495 | 260/423 | 438/848 | 52 |
| . | LMWH (n = 425) . | No LMWH (n = 423) . | Combined (n = 848) . | |
|---|---|---|---|---|
| n/N . | n/N . | n/N . | % . | |
| Thrombophilia | 106/425 | 107/423 | 213/848 | 25 |
| Prior PE | 296/425 | 293/423 | 593/848 | 70 |
| Prior severe PE | 208/304 | 208/304 | 416/608 | 68 |
| Prior SGA <10th percentile | 76/192 | 67/192 | 143/384 | 37 |
| Prior abruption | 91/192 | 90/203 | 181/405 | 45 |
| Prior loss >12 wk | 34/122 | 32/123 | 66/245 | 27 |
| Concomitant ASA use | 178/495 | 260/423 | 438/848 | 52 |
n/N, number (n) with characteristic/total number (N) in treatment group.
Quality assessment of studies included in the meta-analysis
| Reference . | Random sequence generation . | Allocation concealment . | Blinding of participant/personnel . | Blinding of outcome assessors . | Incomplete outcome data . | Selective reporting . | Other bias . |
|---|---|---|---|---|---|---|---|
| 12 | + | + | – | – | + | + | + |
| 13 | + | + | – | + | + | + | + |
| 9 | + | + | – | – | + | – | + |
| 11 | + | + | – | + | + | – | + |
| 10 | + | + | – | + | + | – | + |
| 14 | + | – | – | – | – | – | + |
| Reference . | Random sequence generation . | Allocation concealment . | Blinding of participant/personnel . | Blinding of outcome assessors . | Incomplete outcome data . | Selective reporting . | Other bias . |
|---|---|---|---|---|---|---|---|
| 12 | + | + | – | – | + | + | + |
| 13 | + | + | – | + | + | + | + |
| 9 | + | + | – | – | + | – | + |
| 11 | + | + | – | + | + | – | + |
| 10 | + | + | – | + | + | – | + |
| 14 | + | – | – | – | – | – | + |
(+), low risk of bias; (−), high risk of bias.
In our primary outcome analysis, the composite measure of any PE, abruption, SGA newborn (<10th percentile), or pregnancy loss >20 weeks was significantly reduced by LMWH, with an RR reduction of 0.52 (95% CI, 0.32 to 0.86; P = .01) (Table 4 and Figure 2). Heterogeneity was high (I2 = 69%) for our primary analysis. In a secondary analysis of a composite measure of more severe placenta-mediated pregnancy complications (severe PE as defined by authors) or early-onset PE (<34 weeks), placental abruption, SGA newborn (<5th percentile), or pregnancy loss (>20 weeks), LMWH similarly significantly reduced this more severe composite outcome with an RR reduction of 0.39 (P = .0004) with little heterogeneity noted in this analysis (I2 = 20%). We note that higher-quality trials (Table 3) suggested no treatment effect (Figure 2).
Results of meta-analysis of eligible studies examining prophylactic LMWH vs no LMWH in prevention of pregnancy complications in women with prior placenta-mediated pregnancy complications
| Outcome (LMWH = 288/control = 286) . | Proportion with outcome in the treatment group . | Proportion with outcome in the control group . | RR . | 95% CI . | P . | I2 (%) . | ||
|---|---|---|---|---|---|---|---|---|
| % . | n/N . | % . | n/N . | |||||
| Primary outcome | ||||||||
| Composite: any PE, abruption, SGA child (<10th percentile), or pregnancy loss >20 wk | 18.7 | 67/358 | 42.9 | 127/296 | 0.52 | 0.32-0.86 | .01 | 69 |
| Secondary outcomes | ||||||||
| Composite: severe PE (as defined by authors) or early-onset PE (<34 wk), abruption, SGA child (<5th percentile), or pregnancy loss >20 wk | 7.4 | 22/295 | 22.9 | 59/257 | 0.39 | 0.23-0.65 | .0004 | 20 |
| Any PE | 8.6 | 34/391 | 21.6 | 75/348 | 0.46 | 0.28-0.75 | .0019 | 33 |
| Severe or early PE | 1.7 | 6/352 | 13.4 | 42/313 | 0.16 | 0.07-0.36 | <.0001 | 0 |
| SGA <10th percentile | 10.1 | 39/386 | 29.4 | 96/327 | 0.42 | 0.29-0.59 | <.0001 | 0 |
| SGA <5th percentile | 5.0 | 15/302 | 9.9 | 30/302 | 0.52 | 0.28-0.94 | .03 | 0 |
| Abruption | 0.8 | 3/381 | 2.4 | 9/375 | 0.42 | 0.13-1.4 | .17 | 0 |
| Pregnancy loss <20 wk | 6.7 | 20/297 | 7.5 | 22/294 | 0.89 | 0.50-1.6 | .69 | 0 |
| Pregnancy loss >20 wk | 1.9 | 6/311 | 5.3 | 16/300 | 0.41 | 0.17-1.02 | .06 | 0 |
| Neonatal death | 0.6 | 2/315 | 2.6 | 8/308 | 0.31 | 0.07-1.3 | .10 | 0 |
| Delivery <37 wk | 32.1 | 95/296 | 47.7 | 124/260 | 0.77 | 0.62-0.96 | .02 | 0.4 |
| Delivery <34 wk | 7.9 | 28/356 | 19.3 | 62/322 | 0.45 | 0.30-0.69 | .0002 | 0 |
| Outcome (LMWH = 288/control = 286) . | Proportion with outcome in the treatment group . | Proportion with outcome in the control group . | RR . | 95% CI . | P . | I2 (%) . | ||
|---|---|---|---|---|---|---|---|---|
| % . | n/N . | % . | n/N . | |||||
| Primary outcome | ||||||||
| Composite: any PE, abruption, SGA child (<10th percentile), or pregnancy loss >20 wk | 18.7 | 67/358 | 42.9 | 127/296 | 0.52 | 0.32-0.86 | .01 | 69 |
| Secondary outcomes | ||||||||
| Composite: severe PE (as defined by authors) or early-onset PE (<34 wk), abruption, SGA child (<5th percentile), or pregnancy loss >20 wk | 7.4 | 22/295 | 22.9 | 59/257 | 0.39 | 0.23-0.65 | .0004 | 20 |
| Any PE | 8.6 | 34/391 | 21.6 | 75/348 | 0.46 | 0.28-0.75 | .0019 | 33 |
| Severe or early PE | 1.7 | 6/352 | 13.4 | 42/313 | 0.16 | 0.07-0.36 | <.0001 | 0 |
| SGA <10th percentile | 10.1 | 39/386 | 29.4 | 96/327 | 0.42 | 0.29-0.59 | <.0001 | 0 |
| SGA <5th percentile | 5.0 | 15/302 | 9.9 | 30/302 | 0.52 | 0.28-0.94 | .03 | 0 |
| Abruption | 0.8 | 3/381 | 2.4 | 9/375 | 0.42 | 0.13-1.4 | .17 | 0 |
| Pregnancy loss <20 wk | 6.7 | 20/297 | 7.5 | 22/294 | 0.89 | 0.50-1.6 | .69 | 0 |
| Pregnancy loss >20 wk | 1.9 | 6/311 | 5.3 | 16/300 | 0.41 | 0.17-1.02 | .06 | 0 |
| Neonatal death | 0.6 | 2/315 | 2.6 | 8/308 | 0.31 | 0.07-1.3 | .10 | 0 |
| Delivery <37 wk | 32.1 | 95/296 | 47.7 | 124/260 | 0.77 | 0.62-0.96 | .02 | 0.4 |
| Delivery <34 wk | 7.9 | 28/356 | 19.3 | 62/322 | 0.45 | 0.30-0.69 | .0002 | 0 |
n/N, number (n) with outcome/number (N) in treatment group.
Primary outcome analysis. RR reduction of recurrent placenta-mediated pregnancy complications (any PE, placental abruption, SGA child [<10th percentile] or pregnancy loss >20 weeks) with LMWH in women with prior placenta-mediated pregnancy complications (PE, SGA child [<10th percentile], late pregnancy loss [>12weeks] or placental abruption).
Primary outcome analysis. RR reduction of recurrent placenta-mediated pregnancy complications (any PE, placental abruption, SGA child [<10th percentile] or pregnancy loss >20 weeks) with LMWH in women with prior placenta-mediated pregnancy complications (PE, SGA child [<10th percentile], late pregnancy loss [>12weeks] or placental abruption).
In secondary analyses of individual outcomes (Table 4), any PE, severe PE, SGA <10th percentile, SGA <5th percentile, preterm delivery <37 weeks, and preterm delivery <34 weeks were all importantly and statistically significantly reduced with LMWH with no or little heterogeneity in any of these analyses. Pregnancy loss >20 weeks and neonatal death were importantly but not statistically significantly reduced with LMWH. There were no differences in risk of early pregnancy loss (<20 weeks) with LMWH use.
Discussion
In this systematic review and meta-analysis, we found that LMWH appears to significantly reduce the risk of recurrent placenta-mediated pregnancy complications in women with prior placenta-mediated pregnancy complications. LMWH appears to be a very promising preventative therapy for these common and serious pregnancy complications for which no effective or modestly effective (eg, ASA for PE) currently available secondary prevention strategies exist.8 However, we consider that further multicenter corroborative trials are required before this intervention can be adopted as standard of care because of several limitations in the quality of the evidence (outlined below) and uncertainty regarding which patient subgroups benefit from this costly and inconvenient therapy.
Placenta-mediated pregnancy complications are common and are serious. PE is the most common cause of premature delivery with a resultant impact on fetal and neonatal morbidity and mortality.16 Pregnancy loss, especially recurrent or late pregnancy loss, is a painful event for pregnant women and their families. SGA birth often results in long-term effects in the developing child, including developmental delay and poor school performance and, as adults, children who were SGA are significantly less likely to attain higher academic and professional achievement.17 Effective interventions to reduce the risk of the placenta-mediated pregnancy complications are urgently needed.
Recently, RCTs have been conducted to determine whether LMWH can prevent recurrent early pregnancy loss. Although the findings of these studies are not uniform, they suggest no important treatment effect of anticoagulant prophylaxis.18-24 Interestingly, our meta-analysis similarly demonstrated no effect on reduction of early pregnancy loss (<20 weeks) in patients with prior placenta-mediated pregnancy complications, whereas a statistically nonsignificant reduction in late pregnancy loss (>20 weeks) was observed. It is likely that recurrent pregnancy loss and/or early pregnancy loss have different pathophysiological mechanisms than late placenta-mediated pregnancy complications, and LMWH does not influence these other mechanisms. Perhaps the effect of LMWH is isolated to preventing late complications by preventing placental thrombosis only in the later stages of pregnancy. Regardless, early or recurrent pregnancy loss was not the primary focus of this meta-analysis.
It is also noteworthy that while some studies included only thrombophilic participants,12 others permitted inclusion of thrombophilic participants9,11,13 and still others excluded participants with known thrombophilia.10,14 We felt that it was reasonable to combine these studies because of recent cumulative evidence that does not support an association between thrombophilia and the late placenta-mediated pregnancy complications.25 Pooled results from 10 studies that allowed for analyses including more than 20 000 women showed a small absolute increased risk of pregnancy loss for women with factor V Leiden but not for those with prothrombin G20210A mutation. No association was found for the two mutations with PE, placental abruption, or birth of SGA infants. Hence, it appears unlikely that the effect of LMWH on reducing placenta-mediated pregnancy complications would be modified by thrombophilia. Nonetheless, it is possible that the more severe and less common placenta-mediated pregnancy complications are associated with thrombophilia while the more common milder forms of placenta-mediated pregnancy complications are not.25 By extension, it may be that LMWH prevents recurrent severe placenta-mediated pregnancy complications in the thrombophilic subgroup. Given that thrombophilia may be an effect modifier, future studies that include individual patient meta-analysis or RCTs in thrombophilic women with prior placenta-mediated pregnancy complications should be conducted and may shed further light on this possibility.
Similarly, it may be that prophylactic LMWH is mainly of benefit in the subgroup of patients with prior severe placenta-mediated pregnancy complications and that the beneficial effect is limited to preventing only severe placenta-mediated pregnancy complications. This hypothesis is supported by our finding that the larger RR reductions were observed for the more severe pregnancy complications such as severe composite outcome (RR, 0.39) vs primary milder composite outcome (RR, 0.52), severe PE (RR, 0.16) vs any PE (RR, 0.46), preterm labor <34 weeks (RR, 0.45) vs preterm labor <37 weeks (RR, 0.77), and late pregnancy loss (RR, 0.41) vs early pregnancy loss (RR, 0.89). Notably, LMWH did not have a beneficial effect in the trial that included women with any prior PE (ie, mild or severe)13 while LMWH generally did have a positive effect in the trials that included women with only severe or early-onset PE.9-11 Thus, it is plausible that LMWH is only beneficial in preventing recurrent severe placenta-mediated pregnancy complications.
The major strengths of our systematic review and meta-analysis are that we adhered to all of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines in the conduct and reporting of our systematic review and meta-analysis, and we were able to obtain data clarifications from 5 of 6 authors of the component studies in the meta-analysis. The LMWH dose and timing of initiation of LMWH was relatively homogeneous between studies. All of the component studies were led by academic centers.
Several limitations are worthy of note. First, the placenta-mediated pregnancy complications often overlap, that is, women with one of the prior placenta-mediated pregnancy complications may also have had one or more other placenta-mediated pregnancy complications (eg, women with prior PE may have concomitant abruption and give birth to an SGA newborn). Some of the component studies of our meta-analysis included women with any of the placenta-mediated pregnancy complications whereas others limited inclusion to women with a subset of the placenta-mediated pregnancy complications. Thus, it is difficult to determine whether our findings apply to all patients with placenta-mediated pregnancy complications or to only a limited subset of these patients. Indeed, as noted above, it maybe that the benefit of LMWH will only be to prevent recurrent severe placenta-mediated pregnancy complications. Further trials with limited inclusion criteria or individual patient-level meta-analysis might answer the specific question of which subset benefits or benefits most from LMWH. Second, the primary composite outcome of our meta-analysis and those of many of the component studies are heterogeneous, and we are not able to determine whether LMWH reduces the risk of all or some of the component placenta-mediated pregnancy complications of the composite outcome. This may explain the moderate heterogeneity observed in our primary analysis (I2 = 69%) and the low or no heterogeneity(I2 < 25%) observed in secondary analyses of severe outcomes. Third, ASA was a co-intervention in more than 50% of participants in the component trials, which raises the possibility that the preventative effect of LMWH is biased by ASA use or interacts with ASA use. However, it is important to note that overall ASA use was balanced, and most studies either randomized to ASA use or stratified randomization by ASA use so it is unlikely that ASA use is the sole driver of these findings. Fourth, not all of the component studies were of high quality (as reviewed in Table 3); some did not have independent adjudication of outcomes or adequate allocation concealment. It is noteworthy that it is very challenging, and some argue unethical, to conduct placebo-controlled trials of a long-term injectable drug in pregnancy, and such a design may not be achievable in studies in this area of research. It is also noteworthy and bears emphasizing that the two highest-quality trials12,13 demonstrated no effect on our primary outcome, introducing the possibility that our summary effect is driven by low-quality and possibly biased studies. Fifth, an incomplete data set for the meta-analysis is possible and may have resulted from reporting bias and/or unavailable results in our included studies that we could not resolve through data clarification requests. However, 5 of 6 authors provided data clarifications and were collaborators in this meta-analysis, thereby limiting the amount of missing data. Finally, a large number of participants were recruited in single-center studies9,11,14 or multicenter studies in which the large majority of patients were recruited from 1 center10 ; this raises concerns about external generalizability and potentially introduces selection bias. In light of these limitations, at this time, there is insufficient evidence to support adoption of this intervention in clinical practice. Specifically, high-quality multicenter trials should be conducted with a focus on preventing recurrent severe placenta-mediated pregnancy complications.
In conclusion, LMWH appears to be a promising preventative therapy for recurrent, especially severe, placenta-mediated pregnancy complications. More high-quality multicenter trials should be conducted to confirm this potentially important preventative therapy for a group of common conditions with no effective preventive therapy.
Recommendations
We provide the following weak recommendations based on moderate-quality evidence as suggested by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group (http://www.gradeworkinggroup.org). Weak recommendations are made when the benefits and risks and burdens of therapy are finely balanced, or appreciable uncertainty exists about the magnitude of benefits and risks. Weak recommendations are offered when, across the range of patient values, fully informed patients are liable to make different choices. Moderate-quality evidence is noted when further research is likely to have an important impact on the confidence in the estimate of effect and may change the estimate.
For prevention of recurrent severe placenta-mediated pregnancy complications, we suggest antepartum prophylactic dose LMWH (GRADE 2B–weak recommendation, moderate-quality evidence [inconsistent results]).
For the prevention of recurrent nonsevere placenta-mediated pregnancy complications, we suggest no antepartum prophylactic dose LMWH (GRADE 2B–weak recommendation, moderate-quality evidence [inconsistent results]).
The online version of this article contains a data supplement.
Acknowledgments
We are grateful to the patients who participated in the component clinical trials of the meta-analysis. We also acknowledge the Low-Molecular-Weight Heparin for Placenta Mediated Pregnancy Complications Study Group, which consists of each of the site investigators of the component studies: Drs C. Chauleur, N. Molinari, P. Mares, P. Fabbro-Peray, I. Quere, J.Y. Lefrant, B. Haddad, and M. Dauzat from Nimes; Drs M.G. van Pampus, W.M. Hague, P.D. Bezemerand, J.H. Joosten, and the other Fractionated heparin to Reduce Uterine Insufficiency Trial (FRUIT) investigators; P. Ruggenenti, I. Cetin, G. Pardi, P. Vergani, B. Acaia, F. Facchinetti, G. Battista La Sala, M. Bozzo, S. Rampello, L. Marozio, O. Diadei, G. Gherardi, S. Carminati, G. Remuzzi, P.M. Mannucci, and the Heparin in pregnant women with Adverse Pregnancy outcome to improve the rate of successful PregnancY (HAPPY) study investigators; and Drs P. Garneau, M. David, R. Gauthier, L. Leduc, N. Michon, F. Morin, C. Demers, S.R. Kahn, and L.A. Magee.
M.A.R. was the recipient of a Career Investigator Award from the Heart and Stroke Foundation of Canada and a Chair in Venous Thrombosis and Thrombophilia from the Department of Medicine and Faculty of Medicine, University of Ottawa.
Authorship
Contribution: M.A.R. was the lead investigator for this study, had the initial idea for the study, developed the methods for the systematic review and meta-analysis, and participated in review and selection of included publications, data extraction, and analysis, wrote the first draft of the report, and approved the final version of the paper; M.C. and G.L.G. developed the methods for the systematic review and meta-analysis and participated in review and selection of included publications, data extraction and analysis, and approved the final version of the paper; A.P. assisted in data extraction, data interpretation, reviewed drafts of the paper, and approved the final version of the manuscript; I.M. was the principal investigator for a component study, assisted in data clarification, reviewed drafts of the manuscript, and approved the final version of the paper; E.R. was the principal investigator for a component study, assisted in data clarification, reviewed drafts of the manuscript, and approved the final version of the paper; J.I.P.d.V. was the principal investigator for a component study, assisted in data clarification, reviewed drafts of the manuscript, and approved the final version of the paper; and J.-C.G. was the principal investigator for two component studies, assisted in data clarification, reviewed drafts of the manuscript, and approved the final version of the paper.
Conflict-of-interest disclosure: M.A.R. has received grant funding from Pfizer and Leo Pharma and has served on advisory boards for Sanofi-Aventis; M.C. was a consultant for Leo Pharma and Sanofi-Aventis; J.I.P.d.V. received support for a 2-year investigator grant for the FRUIT-RCT from Pfizer; E.R. received travel grants and consultant honoraria from Leo Pharma; J.-C.G. was a board member of Sanofi-Aventis, LFB Biomanufacturing, and Stago, was a consultant for Sanofi-Aventis, Stago, Leo Pharma, and LFB Biomanufacturing, has received grants from Sanofi-Aventis, Stago, Leo Pharma, LFB Biomanufacturing, and Baxter Healthcare Corporation, and has received lecture fees from Sanofi-Aventis, Stago, Leo Pharma, LFB Biomanufacturing, Bristol-Myers Squibb, Pfizer, Bayer, and Boehringer Ingelheim. The remaining authors declare no competing financial interests.
Correspondence: Marc A. Rodger, The Ottawa Hospital, Centre for Practice-Changing Research, 501 Smyth Rd, Box 201, Ottawa, ON, Canada K1H 8L6; e-mail: mrodger@ohri.ca.

![Figure 2. Primary outcome analysis. RR reduction of recurrent placenta-mediated pregnancy complications (any PE, placental abruption, SGA child [<10th percentile] or pregnancy loss >20 weeks) with LMWH in women with prior placenta-mediated pregnancy complications (PE, SGA child [<10th percentile], late pregnancy loss [>12weeks] or placental abruption).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/6/10.1182_blood-2013-01-478958/4/m_822f2.jpeg?Expires=1764968030&Signature=Qf1fS4eZXN6mAKOuyqio40u4T9qyZxW8-9XUF4qX5bwesLAW53p7LkYHwume4QhgOH4SsGpyAOCvGjYVibCgLFPnaNz7fPjvgaT3pjX2hF0mj8SFMzcFMdi8urGU~0Jjhuhb5l5X2GBo-WdMZBIqh7sYzMgRTAZAgg4mmM-sMKFscpXeyQXlFBAohMLoWWPMozH5Rv7B-RljJLTqfAMIQmxv22d9uKdes-tdy0QLMTPrVOn99f~8ticZbqMW~8NN-wWC~4e9KKl8kBNCpIKBWmFToaqYBjQDho4NAV2x-YgjDq2Kmdz1RPtIfKHffjiEpeqqSuCUPMCHmEdBTZT-HA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)