Key Points
Extracellular nuclear proteins H4 and HMGB1 are highly proinflammatory cytokines.
Inorganic polyP dramatically amplifies proinflammatory responses of H4 and HMGB1 through the RAGE and P2Y1 receptors.
Abstract
The extracellular nuclear proteins, histone H4 (H4) and high mobility group box 1 (HMGB1), released by injured cells during the activation of inflammation and coagulation pathways provoke potent inflammatory responses through interaction with pathogen-related pattern recognition receptors (ie, Toll-like receptors [TLRs] and receptor for advanced glycation end products [RAGE]) present on vascular and innate immune cells. Inorganic polyphosphate (polyP) has emerged as a key modulator of coagulation and inflammation. Here, we demonstrate that polyP binds to both H4 and HMGB1 with high affinity, thereby dramatically potentiating their proinflammatory properties in cellular and in vivo models. By using small interfering RNA knockdowns, pharmacologic inhibitors and extracellular domains of the receptors TLR2, TLR4, RAGE, and P2Y1 as competitive inhibitors, we demonstrate that polyP amplifies H4- and HMGB1-mediated inflammatory signaling in human umbilical vein endothelial cells specifically through interaction with the RAGE and P2Y1 receptors, thereby eliciting intracellular Ca2+ release. Finally, we demonstrate that the natural anticoagulant protease, activated protein C, potently inhibits polyP-mediated proinflammatory effects of both nuclear proteins in cellular and in vivo systems.
Introduction
The normally chromatin-associated nuclear proteins, histones (in particular histone H4 [H4]) and high mobility group box 1 (HMGB1) can act as extracellular cytokines in the pathogenesis of inflammatory disorders.1-3 They can be released into intravascular spaces by cells of the innate immune system, damaged tissues, and necrotic cells in response to bacterial endotoxin and/or trauma.2-4 Activated neutrophils release their nuclear contents, including histones, as extracellular traps to bind and neutralize invading bacteria.5 Elevated H4 and HMGB1 plasma levels correlate with poor prognosis and high mortality in patients with severe sepsis and other inflammatory disorders such as cancer.1,3,6 Proinflammatory signaling cascades initiated by nuclear cytokines through the receptor for advanced glycation end products (RAGE) on platelets and vascular endothelial cells are pivotal in procoagulant and proinflammatory responses.7-10 Toll-like receptor 4 (TLR4) signaling mediated by bacterial membrane lipopolysaccharides (LPS) derived from gram-negative bacteria is implicated in the pathogenesis of severe sepsis.2,3,11,12 Interestingly, LPS potently stimulates HMGB1 and H4 release by endothelial cells, suggesting that LPS can amplify proinflammatory responses indirectly through other pattern recognition receptors including RAGE.10,13 Consistent with their role in the pathogenesis of severe sepsis, pharmacologic inhibition of either H4 or HMGB1 can improve survival in experimental models of endotoxemia, whereas infusion of either H4 or HMGB1 into mice is highly cytotoxic, causing death from multiple organ failure.1-3
Another proinflammatory mediator recently attracting much attention is inorganic polyphosphate (polyP).14 Platelets are rich sources of HMGB1 and polyP, which are stored in dense granules and upon platelet activation, both molecules are secreted into circulation.14,15 Whether polyP and HMGB1 can be secreted as a complex by activated platelets is not known. PolyP stimulates both procoagulant and proinflammatory pathways in vitro and in vivo.14,16 We reported that polyP containing 70 phosphates (polyP-70), similar to the size in platelets, elicits proinflammatory responses by activating nuclear factor κB (NF-κB) in vascular endothelial cells.17 The mechanism of polyP-induced inflammation is poorly understood. PolyP can evoke Ca2+ signals through P2Y1 purinergic receptors (in particular P2Y1) in astrocytes,18 but whether polyP also triggers proinflammatory signaling in endothelial cells through the same pathway is unknown. Furthermore, the possibility that anionic polyP modulates signaling activities of cationic proteins, histones, and HMGB1 during inflammation has not been investigated.
Here, we report the synergistic effect of polyP-70 on proinflammatory functions of H4 and HMGB1, both in cellular and animal models. We show that polyP-70 binds to both proteins with high affinity to dramatically potentiate their proinflammatory signaling effects. By using small interfering RNA knockdowns, pharmacologic inhibitors, and extracellular domains of TLR2, TLR4, RAGE, and P2Y1 as competitive inhibitors, we demonstrate polyP-70 amplifies H4- and HMGB1-mediated proinflammatory signaling pathways through interaction with 2 receptors, RAGE and P2Y1, thereby eliciting a Ca2+ signal and activating NF-κB in endothelial cells. Interestingly, an HMGB1 concentration of 2 to 3 nM in complex with subnanomolar polyP-700 (similar to the size in bacteria) elicits a robust proinflammatory response in endothelial cells. Finally, we show activated protein C (APC) potently inhibits polyP-mediated proinflammatory activity of H4 and HMGB1 in cellular and animal models.
Materials and methods
Reagents
PolyP-70 was a generous gift from Dr James Morrissey (University of Illinois, Urbana, IL). PolyP-700 was purchased from Kerafast. The P2Y1 antagonist, MRS-2279, was purchased from Tocris (Bioscience, United Kingdom). Histone 4 (H4) was from New England Biolabs. Six-week-old male C57BL/6 mice were obtained from The Jackson Laboratory. The complete list of reagents is presented in the supplemental data, available on the Blood Web site.
Recombinant proteins
The recombinant forms of HMGB1 and extracellular domains of all receptors (soluble receptors) soluble TLR2 (sTLR2), soluble TLR4 (sTLR4), soluble RAGE (sRAGE), and soluble P2Y1 (sP2Y1) were expressed in Escherichiacoli using SUMO expression/purification system with a His tag and purified using a combination of Ni-Sepharose and Hi-Trap Q HP column chromatography, as described in the supplemental data.
Cell culture
Permeability assay
Endothelial cell permeability in response to increasing concentrations of HMGB1 (0-80 nM for 16 hours), H4 (0-3.5 µM for 4 hours), and polyP (0-50 µM for 4 hours) was assessed by spectrophotometric measurement of the flux of Evans blue-bound albumin across functional cell monolayer by a modified 2-compartment chamber model as described.13 Cell permeability was also measured in the presence of increasing concentrations of soluble receptors (sRAGE, sTLR2, sTLR4, sP2Y1), and pharmacologic inhibitors thapsigargin (1 µM for 1 hour) and MRS-2279 (50 µM for 1 hour) in the absence and presence of polyP-70 using the same protocol. To investigate the effect of APC, cells were treated with APC for 3 hours before inducing permeability with different stimuli.
Analysis of expression of cell adhesion molecules
The cell surface expression levels of vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), and E-selectin on HUVECs were measured by a whole-cell based enzyme-linked immunosorbent assay as described.13 Briefly, cell monolayers, which were incubated with increasing concentration of H4 (0-3.5 µM, 4 hours), HMGB1 (0-40 nM, 16 hours), and polyP (0-50 µM, 4 hours), were fixed in 1% paraformaldehyde. After washing 3 times, mouse anti–human mAbs to VCAM-1, ICAM-1 and E-selectin were added overnight at 4°C. Cells were then washed and peroxidase-conjugated anti-mouse immunoglobulin G (Sigma) was added for 1 hour. Cells were washed again and developed using o-phenylenediamene substrate (Sigma).
Caspase-8 assay
The activity of caspase-8 was measured using a colorimetric kit (Genescript), according to the manufacturer’s instruction. Briefly, after treating cells with polyP-70 (2.5 µM) in complex with either HMGB1 (10 nM) or H4 (0.44 µM), they were lysed (50 mM HEPES, pH 7.5, 100 mM NaCl, 0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate, 1 mM dithiothreitol, 0.1 mM EDTA) and 50 µL of the cell lysate was incubated for 5 minutes on ice, and then centrifuged at 10 000 × g for 10 minutes at 4°C. Supernatants were incubated with the caspase-8 specific Ac-LEHD-pNA substrate for 4 hours in the dark, and the enzyme activity was measured at 405 nm.
NF-κB activation
After incubating endothelial cells with different stimuli (HMGB1, H4, and polyP-70), cells were lysed with the lysis buffer, and supplemented with protease and phosphatase inhibitor cocktail (Thermo Scientific Pierce, Rockford, IL). The lysate was clarified by centrifugation at 13 000 × g for 10 minutes at 4°C, and the protein content was measured using a protein assay kit (Bio-Rad, Richmond, CA). The protein sample was boiled in sodium dodecyl sulfate loading buffer, separated on a 10% sodium dodecyl sulfate denaturing gel and transferred to a polyvinylidene difluoride membrane followed by its blocking for 2 hours in TBS with Tween 20 buffer (20 mM Tris-HCl, 100 mM NaCl, pH 7.5, 0.1% Tween 20) containing 5% nonfat milk. Western immunoblotting was conducted using primary and secondary antibodies and was then developed with enhanced chemiluminescence reagents.
RNA interference
The cell expression of VCAM-1, ICAM-1, and E-selectin, as well as the permeability barrier function of endothelial cells in response to indicated concentration of H4, HMGB1, and polyP-70 were evaluated after the knockdown of RAGE and P2Y1 expression by pools of target-specific 20- to 25-nucleotide small interfering RNAs (siRNAs) obtained from Santa Cruz Biotechnology Inc., according to the manufacturer’s instruction. A nontargeting 20- to 25-nucleotide siRNA obtained from the same company was used as a negative control.
In vivo permeability and leukocyte migration assays
Six-week-old male C57BL/6 mice were used for in vivo studies after a 5-day acclimatization period. All animals were treated in accordance with the Guidelines for Care and Use of Saint Louis University. The vascular permeability assay was carried out according to previously described methods.17 Briefly, 1% Evans blue dye solution in normal saline was injected intravenously to each mouse, immediately followed by an intraperitoneal injection (µg/g body weight) of H4 (2.5, 5, and 10), HMGB1 (1, 2, and 5), polyP-70 (35, 75, and 150), also mixed concentrations of HMGB1 (1) plus polyP-70 (35) and H4 (2.5) plus polyP-70 (35) or 0.7% acetic acid as a positive control. Thirty minutes later, the mice were sacrificed, and peritoneal exudates were collected (after being washed with 5 mL of normal saline) and centrifuged at 200 × g for 10 minutes. The absorbance of the supernatant was read at 650 nm. The vascular permeability was expressed in terms of dye (µg/mouse), which leaked into the peritoneal cavity, according to a standard curve constructed with Evans blue as described.17
For assessing the leukocyte migration, animals were intraperitoneally injected with the stated concentrations in the previous paragraph of the stimuli dissolved in normal saline. Four hours later, mice were sacrificed and the peritoneal surfaces were washed with 5 mL of normal saline. Next, 20 µL of peritoneal fluid was mixed with 0.38 mL of Turk’s solution (0.01% crystal violet in 3% acetic acid) and the number of leukocytes was counted under a light microscope. Moreover, to investigate the protective effect of APC, recombinant APC (0.2 µg/g of body weight) was injected intravenously 2 hours before the injection of indicated concentrations of inflammatory mediators. In these studies, carboxymethylcellulose-sodium (CMC-Na, 1.5%) was administrated to mice as a positive control for leukocyte migration as described.17 All measurements in mice were performed for 3 mice in each group of the study.
Statistical analysis
Data were expressed as mean ± standard deviation from at least 3 independent experiments. Group data were compared using analysis of variance followed by the Bonferroni post-hoc tests (for comparisons among 3 or more groups) or Student t test, as appropriate. A P value of < .05 was considered statistically significant.
Results
HMGB1, H4, and polyP-70 enhance barrier permeability in endothelial cells
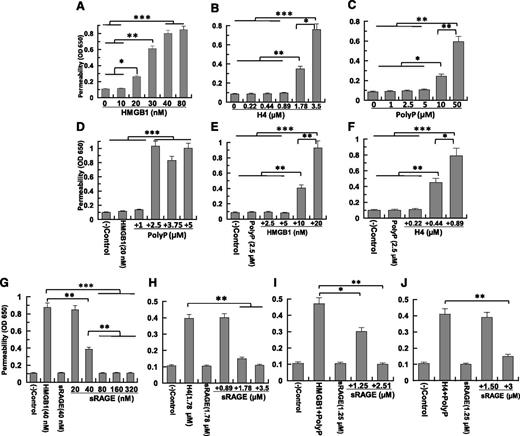
Endothelial cells normally render the vasculature impermeable to circulating immune cells and plasma proteins. During inflammation, the integrity of the endothelial cell monolayer is compromised, allowing leakage of plasma and inflammatory cells into subendothelial tissues. We used a barrier permeability assay to measure proinflammatory signaling responses of polyP and nuclear cytokines. HMGB1, H4, and polyP-70 enhance the barrier permeability of endothelial cells in a concentration-dependent manner (Figure 1A-C). We used both primary and transformed HUVECs in permeability assays obtaining indistinguishable results (supplemental Figure 2). Minimum concentrations for barrier disruption were: 20 nM (0.5 µg/mL) for HMGB1, 1.78 µM (20 µg/mL) for H4, and 10 µM (1 µg/mL) for polyP-70 (polyP concentration expressed in terms of phosphate monomer, unless otherwise indicated).14 We hypothesized polyP-70 binds HMGB1 and/or histones and modulates their proinflammatory activities. To assess whether polyP-70 potentiates HMGB1 activity, we treated endothelial cells with a threshold HMGB1 concentration (10-20 nM) together with a low concentration of polyP-70 (1-5 µM) that by itself exhibited no activity. Surprisingly, 2.5 µM polyP-70 (36 nM if calculated based on the polymer molecular weight [70 × 102 = 7140 Da]), far below that required to induce permeability, dramatically enhanced the barrier-disruptive effect of a low concentration of HMGB1 (Figure 1D-E). Similarly, an H4 concentration (0.44 µM, ∼5 µg/mL) having no signaling activity alone (Figure 1B) induces a potent barrier-disruptive effect in the presence of 2.5 µM polyP-70 (Figure 1F). To test whether polyP-70 directly binds to nuclear cytokines, we monitored interaction of these proteins with polyP-70 by measuring fluorescence anisotropy of labeled polyP at equilibrium upon nuclear cytokine binding. PolyP-70 binding to HMGB1 and H4 results in enhanced anisotropy of polyP, yielding dissociation constants of 220 nM and 237 nM, respectively (supplemental Figure 4A,C). These results suggest polyP-70 binds to both nuclear proteins with high affinity to promote their proinflammatory functions.
Proinflammatory signaling by nuclear cytokines in the absence and presence of polyP-70. (A) Cell permeability in response to increasing concentration of HMGB1 (16 hours) was measured by spectrophotometric measurement of the flux of Evans blue-bound albumin across functional endothelial cell monolayers using a modified 2-compartment chamber model. (B-C) The same as (A) except that the barrier disruptive effect of increasing concentrations of either H4 (4 hours) or polyP-70 (4 hours), respectively, were monitored. (D) The same as (A), except that the barrier-disruptive effect of different increasing concentrations of polyP-70 in complex with a fixed concentration of HMGB1 (20 nM) was monitored. (E-F) The same as (D), except that the barrier-disruptive effect of different concentrations of HMGB1 and H4, respectively, was monitored in the presence of a fixed concentration of polyP-70 (2.5 µM). (G-J) The same as other panels, except that the competitive effect of sRAGE on the barrier disruptive effect of HMGB1 (40 nM) and H4 (1.78 µM) was monitored in either the absence (G-H) or presence of polyP-70 (I-J). In the presence of polyP (2.5 µM), the concentrations of nuclear cytokines were reduced to 10 nM for HMGB1 and 0.44 µM for H4 (I-J). All results are shown as mean ± standard deviation of 3 different experiments. OD, optical density. *P < .05; **P < .01; ***P < .001.
Proinflammatory signaling by nuclear cytokines in the absence and presence of polyP-70. (A) Cell permeability in response to increasing concentration of HMGB1 (16 hours) was measured by spectrophotometric measurement of the flux of Evans blue-bound albumin across functional endothelial cell monolayers using a modified 2-compartment chamber model. (B-C) The same as (A) except that the barrier disruptive effect of increasing concentrations of either H4 (4 hours) or polyP-70 (4 hours), respectively, were monitored. (D) The same as (A), except that the barrier-disruptive effect of different increasing concentrations of polyP-70 in complex with a fixed concentration of HMGB1 (20 nM) was monitored. (E-F) The same as (D), except that the barrier-disruptive effect of different concentrations of HMGB1 and H4, respectively, was monitored in the presence of a fixed concentration of polyP-70 (2.5 µM). (G-J) The same as other panels, except that the competitive effect of sRAGE on the barrier disruptive effect of HMGB1 (40 nM) and H4 (1.78 µM) was monitored in either the absence (G-H) or presence of polyP-70 (I-J). In the presence of polyP (2.5 µM), the concentrations of nuclear cytokines were reduced to 10 nM for HMGB1 and 0.44 µM for H4 (I-J). All results are shown as mean ± standard deviation of 3 different experiments. OD, optical density. *P < .05; **P < .01; ***P < .001.
RAGE mediates polyP-enhanced barrier-disruptive effect of HMGB1 and H4
Proinflammatory signaling by HMGB1 and H4 is thought to be mediated through pattern recognition receptors, RAGE, TLR2, and TLR4.7-13 We evaluated the contribution of each receptor to polyP-mediated proinflammatory signaling by HMGB1 and H4 using recombinant extracellular domains of RAGE, TLR2, and TLR4 as competitive inhibitors of nuclear cytokine signaling in permeability assays. Recombinant proteins were purified to reasonable homogeneity (supplemental Figure 1). The RAGE extracellular domain (sRAGE) potently inhibits HMGB1 and H4 signaling in endothelial cells (Figure 1G-J). An sRAGE concentration of only ∼2 molar in excess of HMGB1 or H4 effectively inhibits barrier-disruptive effects of both nuclear cytokines in the absence of polyp-70 (Figure 1G-H), but sRAGE inhibition is not as efficient in the presence of polyP-70 (Figure 1I-J). Interestingly, while soluble forms of both TLR2 and TLR4 (ie, sTLR2 and sTLR4) are equally effective in inhibiting HMGB1 signaling (supplemental Figure 3A), neither protein competes with HMGB1 or H4 signaling in the presence of polyP-70 (supplemental Figure 3B-C), suggesting polyP-70 primarily binds to RAGE to promote the proinflammatory signaling by nuclear proteins. Supporting this hypothesis, we found sRAGE binds to polyP-70 with a dissociation constant of 100 nM, whereas neither sTLR2 nor sTLR4 exhibits detectable affinity for polyP-70 (supplemental Figure 4D).
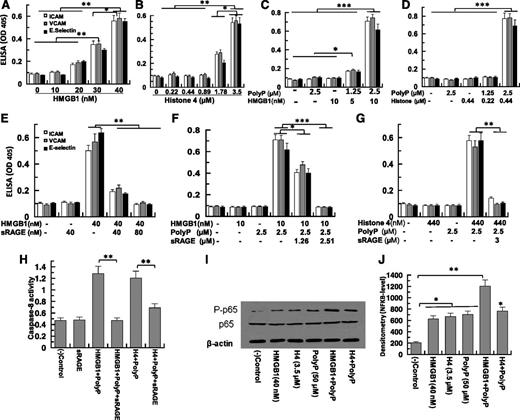
PolyP-70 promotes CAM expression and apoptosis by HMGB1 and H4 through RAGE
Both HMGB1 and H4 induce expression of ICAM-1, VCAM-1, and E-selectin on endothelial cells (Figure 2A-B). PolyP-70 potently promotes HMGB1- and H4-mediated expression of cell adhesion molecules (CAMs) (Figure 2C-D). Thus, a polyP-70 concentration (2.5 µM) exhibiting no signaling function by itself dramatically upregulates expression of all 3 CAMs by low concentrations of nuclear cytokines that are also inactive by themselves (10 nM HMGB1 and 0.44 µM H4) (Figure 2C-D). Consistent with RAGE being the primary receptor for HMGB1 and H4 signaling, sRAGE effectively inhibits polyP-70-mediated induction of CAM expression by both nuclear cytokines (Figure 2E-G). sRAGE also inhibits the pro-apoptotic effect of polyP-70-mediated HMGB1 and H4, as evidenced by inhibiting caspase-8 activation by nuclear cytokines (Figure 2H). These proinflammatory responses appear to be mediated through polyP-70 promoting NF-κB activation by nuclear cytokines (Figure 2I-J).
HMGB1- and H4-mediated expression of CAMs in HUVECs in the absence and presence of polyP-70. (A) Confluent endothelial cells were incubated with increasing concentrations of HMGB1 (10-40 nM for 16 hours) followed by measuring the cell surface expression of ICAM-1 (white bar), VCAM-1 (gray bar), and E-selectin (black bar) by a cell-based enzyme-linked immunosorbent assay (ELISA). (B) The same as (A), except that H4 (0.22-3.5 µM) was used for endothelial cell activation. (C) The same as (A), except that polyP-mediated amplification of signaling by low concentrations of HMGB1 (5-10 nM) was monitored. (D) The same as (A), except that polyP-mediated amplification of signaling by low concentrations of H4 (0.22-0.44 µM) was monitored. (E) The same as (A), except that the competitive effect of sRAGE on CAM expression by HMGB1 (40 nM) was monitored. (F) The same as (E), except that the competitive effect of sRAGE on the polyP-mediated amplification of signaling by a low concentration of HMGB1 (10 nM) was monitored. (G) The same as (E), except that the competitive effect of sRAGE on the polyP-mediated amplification of signaling by a low concentration of H4 (0.44 µM) was monitored. (H) Analysis of caspase-8 activity by a colorimetric assay. (I) Analysis of NF-κB activation by different stimuli (lane 1, buffer control; lane 2, 40 nM HMGB1; lane 3, 3.5 µM H4; lane 4, 50 µM polyP; lane 5, 2.5 µM polyP-70 + 10 nM HMGB1; lane 6, 2.5 µM polyP-70 + 0.44 µM H4). (J) Densitometric analysis of NF-κB data in (I). All results are shown as mean ± standard deviation of 3 different experiments. *P < .05; **P < .01; ***P < .001.
HMGB1- and H4-mediated expression of CAMs in HUVECs in the absence and presence of polyP-70. (A) Confluent endothelial cells were incubated with increasing concentrations of HMGB1 (10-40 nM for 16 hours) followed by measuring the cell surface expression of ICAM-1 (white bar), VCAM-1 (gray bar), and E-selectin (black bar) by a cell-based enzyme-linked immunosorbent assay (ELISA). (B) The same as (A), except that H4 (0.22-3.5 µM) was used for endothelial cell activation. (C) The same as (A), except that polyP-mediated amplification of signaling by low concentrations of HMGB1 (5-10 nM) was monitored. (D) The same as (A), except that polyP-mediated amplification of signaling by low concentrations of H4 (0.22-0.44 µM) was monitored. (E) The same as (A), except that the competitive effect of sRAGE on CAM expression by HMGB1 (40 nM) was monitored. (F) The same as (E), except that the competitive effect of sRAGE on the polyP-mediated amplification of signaling by a low concentration of HMGB1 (10 nM) was monitored. (G) The same as (E), except that the competitive effect of sRAGE on the polyP-mediated amplification of signaling by a low concentration of H4 (0.44 µM) was monitored. (H) Analysis of caspase-8 activity by a colorimetric assay. (I) Analysis of NF-κB activation by different stimuli (lane 1, buffer control; lane 2, 40 nM HMGB1; lane 3, 3.5 µM H4; lane 4, 50 µM polyP; lane 5, 2.5 µM polyP-70 + 10 nM HMGB1; lane 6, 2.5 µM polyP-70 + 0.44 µM H4). (J) Densitometric analysis of NF-κB data in (I). All results are shown as mean ± standard deviation of 3 different experiments. *P < .05; **P < .01; ***P < .001.
PolyP-70 induces Ca2+ signaling in endothelial cells through P2Y1
PolyP mediates astrocyte communication through the P2Y1 receptor and release of Ca2+ from internal stores.18 We took 4 different approaches to determine whether proinflammatory signaling by polyP-70 occurs through the same mechanism. First, we tested the activity of the potent Ca2+ pump inhibitor thapsigargin in endothelial cell permeability induced by polyP-70, HMGB1, and H4. Thapsigargin blocks intracellular signaling induced by all 3 stimuli, both individually and in combination with polyP-70 (Figure 3A). Second, we conducted nuclear cytokine-mediated permeability assays using the P2Y1 antagonist MRS-2279.18 MRS-2279 inhibits the barrier-disruptive activity of polyP-70 (50 µM), as well as polyP-70 (2.5 µM)-mediated barrier-disruptive effects of H4 (0.44 µM) and HMGB1 (10 nM), but not the effect of either H4 (3.5 µM) or HMGB1 (40 nM) alone (Figure 3B). Because polyP-70 signals through both RAGE and P2Y1, MRS-2279 only partially inhibits polyP-70 signaling (Figure 3B). Third, we expressed and purified the 51-residue P2Y1 extracellular domain (sP2Y1) from E coli and evaluated its competitive effect in signaling assays. sP2Y1 has no competitive effect on HMGB1 signaling and only partially inhibits polyP-70 signaling (Figure 3C-D), confirming the inference that polyP-70 signals through both RAGE and P2Y1, that the effect is additive and that blocking the latter receptor only partially inhibits polyP-70 signaling. In contrast, sP2Y1 effectively inhibits polyP-mediated signaling of both HMGB1 and H4, suggesting polyP interaction with P2Y1 is essential in promoting nuclear cytokine signaling. sP2Y1 effectively inhibits polyP-mediated nuclear cytokines induction of VCAM and E-selectin (Figure 3E-F). These results clearly suggest proinflammatory signaling of these molecules at low concentrations, which requires polyP-70 interaction with P2Y1. Results presented as follows, in the fourth approach, P2Y1 knockdown, further confirm this hypothesis.
PolyP-70-mediated proinflammatory signaling by nuclear cytokines is mediated by a Ca2+signal through interaction with P2Y1. (A) Cell permeability in response to HMGB1 (40 nM), H4 (1.78 μM), and polyP-70 (50 μM) was evaluated in the absence (gray bar) and presence of thapsigargin (black bar) as described in Materials and methods. (B) The same as (A), except that the cell permeability in response to HMGB1 (40 nM), H4 (3.5 μM), polyP-70 (50 μM), polyP-70 (2.5 μM) + HMGB1 (10 nM), and polyP-70 (2.5 μM) + H4 (0.44 μM) was evaluated in the absence (gray bar) and presence (black bar) of the P2Y1 antagonist MRS-2279. (C) The same as (A), except that the competitive effect of sP2Y1 (25-50 μM) on cell permeability in response to HMGB1 (40 nM) and polyP-70 (50 μM) was evaluated. (D) The same as (C), except that the competitive effect of sP2Y1 (1.25-3 μM) on a low concentration of polyP-70 (2.5 μM)-mediated cell permeability by a low concentration of either HMGB1 (10 nM) or H4 (0.44 μM) was evaluated. (E) The same as (C), except that the competitive effect of sP2Y1 on the expression of either VCAM-1 (gray bar) or E-selectin (black bar) in response to HMGB1 (40 nM) and polyP-70 (50 μM) was evaluated. (F) The same as (D), except that the competitive effect of sP2Y1 (1.25-3 μM) on the polyP-70 (2.5 μM)-mediated expression of either VCAM-1 (gray bar) or E-selectin (black bar) by a low concentration of either HMGB1 (10 nM) or H4 (0.44 μM) was evaluated. All results are shown as mean ± standard deviation of 3 different experiments. *P < .05; **P < .01; ***P < .001.
PolyP-70-mediated proinflammatory signaling by nuclear cytokines is mediated by a Ca2+signal through interaction with P2Y1. (A) Cell permeability in response to HMGB1 (40 nM), H4 (1.78 μM), and polyP-70 (50 μM) was evaluated in the absence (gray bar) and presence of thapsigargin (black bar) as described in Materials and methods. (B) The same as (A), except that the cell permeability in response to HMGB1 (40 nM), H4 (3.5 μM), polyP-70 (50 μM), polyP-70 (2.5 μM) + HMGB1 (10 nM), and polyP-70 (2.5 μM) + H4 (0.44 μM) was evaluated in the absence (gray bar) and presence (black bar) of the P2Y1 antagonist MRS-2279. (C) The same as (A), except that the competitive effect of sP2Y1 (25-50 μM) on cell permeability in response to HMGB1 (40 nM) and polyP-70 (50 μM) was evaluated. (D) The same as (C), except that the competitive effect of sP2Y1 (1.25-3 μM) on a low concentration of polyP-70 (2.5 μM)-mediated cell permeability by a low concentration of either HMGB1 (10 nM) or H4 (0.44 μM) was evaluated. (E) The same as (C), except that the competitive effect of sP2Y1 on the expression of either VCAM-1 (gray bar) or E-selectin (black bar) in response to HMGB1 (40 nM) and polyP-70 (50 μM) was evaluated. (F) The same as (D), except that the competitive effect of sP2Y1 (1.25-3 μM) on the polyP-70 (2.5 μM)-mediated expression of either VCAM-1 (gray bar) or E-selectin (black bar) by a low concentration of either HMGB1 (10 nM) or H4 (0.44 μM) was evaluated. All results are shown as mean ± standard deviation of 3 different experiments. *P < .05; **P < .01; ***P < .001.
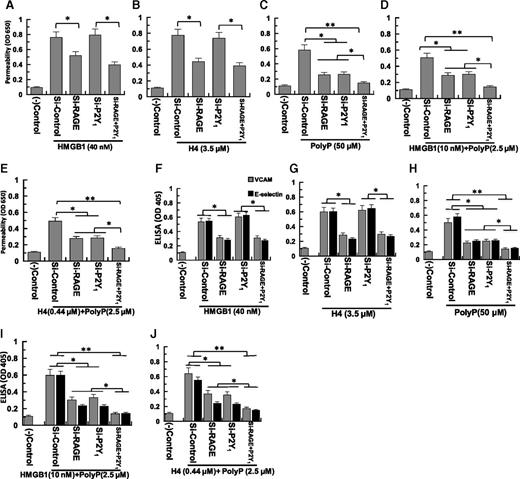
PolyP-70 promotes proinflammatory effects of HMGB1 and H4 through P2Y1 and RAGE
Competitive inhibitors of RAGE (sRAGE) and P2Y1 (MRS-2279 and sP2Y1) inhibit proinflammatory activities of both polyP and polyP-mediated nuclear cytokines, suggesting polyP-70 interacts with both receptors. We tested polyP-70-, HMGB1-, and H4-mediated signaling in endothelial cells knocked-down for RAGE and P2Y1, using barrier permeability (Figure 4A-E) and CAM expression assays (VCAM-1 or E-selectin) (Figure 4F-J). In both assays, knockdown of RAGE, but not of P2Y1, inhibits proinflammatory signaling by HMGB1 (40 nM) and H4 (3.5 µM) in both permeability (Figure 4A-B) and CAM assays (Figure 4F-G). By contrast, knockdown of either P2Y1 or RAGE partially inhibits proinflammatory signaling when polyP-70 is at high concentration by itself (50 µM) (Figure 4C,H) or at low concentration (2.5 µM) together with low HMGB1 (10 nM) or H4 (0.44 µM) concentrations (Figure 4D-E). Double knockdown nearly completely inhibits polyP-mediated proinflammatory effects of both cytokines (Figure 4I-J). These results implicate both receptors in the mechanisms of polyP-70 and polyP-70-mediated potentiation of HMGB1 and H4 signaling. RT-PCR analysis indicated ∼70% to 75% knockdown efficiency by siRNAs under experimental conditions, as described in the legend of Figure 4.
Effect of siRNA knockdown of the RAGE and P2Y1receptors on the HMGB1- and H4-mediated cell permeability in the absence and presence of polyP-70. (A-C) Confluent endothelial cells were transfected with the control siRNA (1 µg for 3 days) or siRNA (1 µg for 3 days) specific for RAGE and P2Y1, individually or in combination of 2 before monitoring cell permeability in response to HMGB1 (40 nM), H4 (3.5 µM), and polyP-70 (50 µM). (D-E) The same as (A-B), except that permeability in response to a low concentration of either HMGB1 (10 nM) or H4 (0.44 μM) was evaluated in the presence of a low concentration of polyP-70 (2.5 μM). (F-J) The same as (A-E), except that the effect of siRNA knockdown of RAGE and P2Y1 on the expression of either VCAM-1 (gray bar) or E-selectin (black bar) in response to different stimuli was studied. All results are shown as mean ± standard deviation of 3 different experiments. *P < .05; **P < .01.
Effect of siRNA knockdown of the RAGE and P2Y1receptors on the HMGB1- and H4-mediated cell permeability in the absence and presence of polyP-70. (A-C) Confluent endothelial cells were transfected with the control siRNA (1 µg for 3 days) or siRNA (1 µg for 3 days) specific for RAGE and P2Y1, individually or in combination of 2 before monitoring cell permeability in response to HMGB1 (40 nM), H4 (3.5 µM), and polyP-70 (50 µM). (D-E) The same as (A-B), except that permeability in response to a low concentration of either HMGB1 (10 nM) or H4 (0.44 μM) was evaluated in the presence of a low concentration of polyP-70 (2.5 μM). (F-J) The same as (A-E), except that the effect of siRNA knockdown of RAGE and P2Y1 on the expression of either VCAM-1 (gray bar) or E-selectin (black bar) in response to different stimuli was studied. All results are shown as mean ± standard deviation of 3 different experiments. *P < .05; **P < .01.
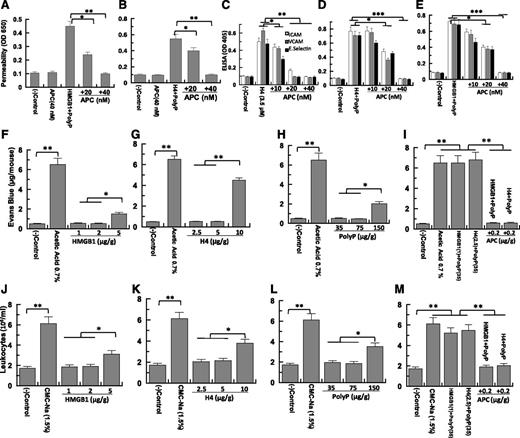
APC inhibits proinflammatory effects of polyP-70, HMGB1, and H4 in vitro and in vivo
APC is a natural anticoagulant with cytoprotective and anti-inflammatory effects both in vitro and in vivo.10,19-21 APC abrogates polyP-70-mediated proinflammatory signaling by HMGB1 and H4 in both permeability (Figure 5A-B) and CAM expression assays (Figure 5C-E). HMGB1 and H4 combined with low polyP-70 concentrations were injected intraperitoneally into mice, followed by measuring vascular permeability based on extravasation of bovine serum albumin-bound Evans blue dye from plasma into the peritoneal cavity. Minimum concentrations of 10 µg/g (H4), 5 µg/g (HMGB1), and 150 µg/g (polyP-70) are required to induce vascular permeability in mice (Figure 5F-H). PolyP-70 dramatically potentiates proinflammatory effects of nuclear cytokines; low polyP-70 concentrations (35 µg/g) dramatically increase endothelium leakiness induced by low HMGB1 (1 µg/g) or H4 (2.5 µg/g) concentrations (Figure 5I) that are inactive when administered alone. Similarly, polyP-70 markedly enhances HMGB1- and H4-mediated binding of leukocytes to the vascular endothelium and their subsequent migration to the peritoneal cavity (Figure 5J-M). The intravenous administration of APC (0.2 µg/g) abrogates proinflammatory effects of polyP-70 + nuclear cytokines in both in vivo assays (Figure 5I,M). Previous results have indicated that APC can exert a protective activity through the cleavage of H4.1 Although a protective effect from the APC cleavage of H4 cannot be ruled out in this model, nevertheless, the APC cleavage of HMGB1 cannot account for its protective mechanism because we have demonstrated that a high concentration of APC is unable to cleave to HMGB1.13
Effect of APC on the nuclear cytokine-mediated proinflammatory responses in the absence and presence of polyP-70 in cellular and animal models. (A) Confluent endothelial cells were treated with APC (20-40 nM) before measuring cell permeability in response to polyP-70 (2.5 µM) + HMGB1 (10 nM). (B) The same as (A), except that the effect of APC on the polyP-70-mediated permeability in response to H4 (0.44 µM) was measured. (C) The effect of APC (10-40 nM) on the H4 (3.5 µM)-mediated expression of either VCAM-1 (gray bar) or E-selectin (black bar) was studied. (D) The same as (C), except that the effect of APC on the polyP-70 (2.5 μM)-mediated expression of ICAM-1 (white bar), VCAM-1 (gray bar), and E-selectin (black bar) by H4 (0.44 µM) was monitored. (E) The same as (D), except that the effect of APC on the polyP-70 (2.5 μM)-mediated expression of ICAM-1 (white bar), VCAM-1 (gray bar), and E-selectin (black bar) by HMGB1 (10 nM) was monitored. (F-H) In vivo analysis of the effect of HMGB1, H4, and polyP-70 on vascular leakage. Mice (n = 3 for every experiment) were intravenously injected with 1% bovine serum albumin-bound Evans blue dye followed by an immediate intraperitoneal injection of HMGB1 (1-5 µg/g body weight), H4 (2.5-10 µg/g body weight), or polyP-70 (35-150 µg/g body weight) with 0.7% acetic acid as a positive control. Vascular permeability was determined from the extent of extravasation of Evans blue to the peritoneal cavity as described in the Materials and methods section. (I) The same as above (F-H), except that the effect of APC (0.2 µg/g body weight) on in vivo permeability was monitored in response to polyP-70 (35 µg/g body weight) plus either HMGB1 (1 µg/g body weight) or H4 (2.5 µg/g body weight). (J-M) The same as (F-I), except that the in vivo analysis of the effect of polyP-70, HMGB1, and H4 on the migration of leukocytes to peritoneal cavity in the absence and presence of APC treatment was studied. *P < .05; **P < .01; ***P < .001.
Effect of APC on the nuclear cytokine-mediated proinflammatory responses in the absence and presence of polyP-70 in cellular and animal models. (A) Confluent endothelial cells were treated with APC (20-40 nM) before measuring cell permeability in response to polyP-70 (2.5 µM) + HMGB1 (10 nM). (B) The same as (A), except that the effect of APC on the polyP-70-mediated permeability in response to H4 (0.44 µM) was measured. (C) The effect of APC (10-40 nM) on the H4 (3.5 µM)-mediated expression of either VCAM-1 (gray bar) or E-selectin (black bar) was studied. (D) The same as (C), except that the effect of APC on the polyP-70 (2.5 μM)-mediated expression of ICAM-1 (white bar), VCAM-1 (gray bar), and E-selectin (black bar) by H4 (0.44 µM) was monitored. (E) The same as (D), except that the effect of APC on the polyP-70 (2.5 μM)-mediated expression of ICAM-1 (white bar), VCAM-1 (gray bar), and E-selectin (black bar) by HMGB1 (10 nM) was monitored. (F-H) In vivo analysis of the effect of HMGB1, H4, and polyP-70 on vascular leakage. Mice (n = 3 for every experiment) were intravenously injected with 1% bovine serum albumin-bound Evans blue dye followed by an immediate intraperitoneal injection of HMGB1 (1-5 µg/g body weight), H4 (2.5-10 µg/g body weight), or polyP-70 (35-150 µg/g body weight) with 0.7% acetic acid as a positive control. Vascular permeability was determined from the extent of extravasation of Evans blue to the peritoneal cavity as described in the Materials and methods section. (I) The same as above (F-H), except that the effect of APC (0.2 µg/g body weight) on in vivo permeability was monitored in response to polyP-70 (35 µg/g body weight) plus either HMGB1 (1 µg/g body weight) or H4 (2.5 µg/g body weight). (J-M) The same as (F-I), except that the in vivo analysis of the effect of polyP-70, HMGB1, and H4 on the migration of leukocytes to peritoneal cavity in the absence and presence of APC treatment was studied. *P < .05; **P < .01; ***P < .001.
Longer chain polyP-700 dramatically promotes proinflammatory signaling by HMGB1 and H4
Finally, we tested the proinflammatory effect of polyP-700, which can be synthesized by bacteria and released to circulation during infection.14,22 Strikingly, we found that ∼40-fold lower polyP-700 concentration was sufficient to dramatically potentiate proinflammatory signaling by both HMGB1 and H4 in the permeability assay (Figure 6A-E). Thus, in contrast to 2.5 µM polyP-70, a polyP-700 concentration of 0.067 µM (0.1-0.2 nM if calculated based on the polymer molecular weight [700 × 102 = 71 400 Da]) effectively amplified signaling of low HMGB1 (2.5-5 nM, 0.067-0.125 µg/mL) (Figure 6D) and H4 (88 nM, 1 µg/mL) (Figure 6E) concentrations in this assay. Thus, longer chain polyP molecules secreted by bacteria could dramatically upregulate inflammation during infection. Each polyP-700 monomer binds to 5 HMGB1, possibly explaining its dramatic proinflammatory effect (supplemental Figure 4B). These findings may have important ramifications for the mechanism of polyP-mediated amplification of inflammatory signaling responses during bacteria-induced severe sepsis.
Analysis of longer chain polyP-700-mediated proinflammatory signaling effects of HMGB1 and H4 in the permeability assay. (A) Endothelial cell permeability in response to increasing concentrations of polyP-700 (0.5-10 μM) was measured. (B) Endothelial cell permeability in response to HMGB1 (10 nM) was measured in the presence of increasing concentrations of polyP-700 (0.035-0.5 μM) was measured. (C) Endothelial cell permeability in response to H4 (0.44 μM) was measured in the presence of increasing concentrations of polyP-700 (0.035-0.5 μM). (D) Endothelial cell permeability in response to increasing concentrations of HMGB1 (1.25-10 nM) was measured in the presence of a fixed concentration of polyP-700 (0.067 μM). (E) Endothelial cell permeability in response to increasing concentrations of H4 (44-440 nM) was measured in the presence of a fixed concentration of polyP-700 (0.067 μM). *P < .05; **P < .01; ***P < .001.
Analysis of longer chain polyP-700-mediated proinflammatory signaling effects of HMGB1 and H4 in the permeability assay. (A) Endothelial cell permeability in response to increasing concentrations of polyP-700 (0.5-10 μM) was measured. (B) Endothelial cell permeability in response to HMGB1 (10 nM) was measured in the presence of increasing concentrations of polyP-700 (0.035-0.5 μM) was measured. (C) Endothelial cell permeability in response to H4 (0.44 μM) was measured in the presence of increasing concentrations of polyP-700 (0.035-0.5 μM). (D) Endothelial cell permeability in response to increasing concentrations of HMGB1 (1.25-10 nM) was measured in the presence of a fixed concentration of polyP-700 (0.067 μM). (E) Endothelial cell permeability in response to increasing concentrations of H4 (44-440 nM) was measured in the presence of a fixed concentration of polyP-700 (0.067 μM). *P < .05; **P < .01; ***P < .001.
Discussion
We report for the first time that polyP binds nuclear cytokines HMGB1 and H4 to dramatically amplify their proinflammatory signaling through 2 cell surface receptors, RAGE and P2Y1. PolyP-mediated amplification of HMGB1 and H4 effects is mediated through increased intracellular Ca2+ and subsequent NF-κB pathway activation, because thapsigargin abrogates barrier-disruptive effects of both nuclear cytokines. The hypothesis that polyP amplifies nuclear cytokine-mediated proinflammatory signaling through 2 receptors is supported by observations that sRAGE, as a competitive inhibitor of RAGE, and MRS-2279 and sP2Y1, as competitive inhibitors of P2Y1, effectively abrogate polyP-70-mediated signaling by both HMGB1 and H4. The observation that competitive inhibitors of P2Y1 only partially inhibit signaling activities of high polyP-70 concentrations further implicates polyP-70 signaling through both RAGE and P2Y1 receptors. Knockdown of both receptors is required to effectively inhibit polyP-70 signaling activity, which further supports this hypothesis.
HMGB1 exerts its proinflammatory effect through RAGE and/or TLRs (in particular TLR2 and TLR4).7-9 That knockdown of RAGE, but not P2Y1, inhibits both HMGB1 and H4 signaling and further supports the central role of RAGE in nuclear cytokine signaling; however, it envisions no role for P2Y1 in signaling by these inflammatory mediators. This is also consistent with results obtained with specific P2Y1 antagonists, MRS-2279 and sP2Y1, which only modulate signaling by polyP-70, but not by either HMGB1 or H4. By contrast, optimal signaling by either polyP-70 alone (50 µM) or in combination (2.5 µM) with a low HMGB1/H4 concentration requires both RAGE and P2Y1, suggesting polyP-70 interaction with both receptors is essential for direct signaling, as well as the ability to amplify signaling by the 2 nuclear cytokines. The additive stimulatory effects further suggest RAGE and P2Y1 mediate polyP-70 signaling through 2 independent pathways.
Because a high polyP-70 concentration (50 µM) is required to elicit proinflammatory signaling in cell-based assays, the physiological significance of platelet-derived polyP signaling by itself is unknown. By contrast, proinflammatory responses observed with a mixture of low polyP-70 concentration (2.5 µM) and nuclear cytokines in the nM range is highly physiologically relevant, because such concentrations are reportedly present in circulation when inflammatory and procoagulant pathways are activated during infection, injury, and/or various other proinflammatory conditions.14,15 Interaction of polyP-70 with P2Y1 is a prerequisite for amplification of HMGB1- and H4-mediated proinflammatory responses, because both antagonists of P2Y1, MRS-2279, and sP2Y1, which modulate signaling only by polyP-70, but not by either HMGB1 or H4, effectively inhibit polyP-potentiated signaling of both nuclear cytokines.
RAGE signaling plays a critical role in the pathogenesis of various acute and chronic proinflammatory disorders including severe sepsis, diabetes, atherosclerosis, neurodegeneration, and cancer.7-10 RAGE null mice (or wild-type mice treated with sRAGE) exhibit increased survival in various inflammatory animal models.23-25 Our findings strongly suggest polyP, which may be released at high levels during inflammation and coagulation by activated platelets and endothelial cells, is crucial in amplifying RAGE-associated proinflammatory signaling in these disease processes. The physiological significance of polyP-mediated amplification of RAGE signaling by nuclear cytokines was evidenced by findings that peritoneal injection of low polyP-70 concentrations in combination with low concentrations of either HMGB1 or H4 dramatically enhances vascular barrier permeability and accumulation of activated immune cells in the peritoneal cavity of mice. Proinflammatory effects are mediated by the binary complex of polyP-70 and HMGB1/H4, because neither polyP-70 nor the cytokine alone elicit any inflammatory response in this model.
APC was used for more than a decade to treat severe sepsis before being withdrawn due to its lack of mortality-reducing effect in a recent clinical trial.26,27 Our results suggest APC can effectively inhibit polyP-70-mediated proinflammatory signaling elicited by nuclear cytokines in both cellular and animal models. This suggests APC can exert a protective effect through inhibition of RAGE signaling, although a high concentration of APC is required to achieve an optimal effect. Given the bleeding side effect of high APC concentrations, APC derivatives lacking anticoagulant activity, but possessing normal anti-inflammatory activity will be required to assess the therapeutic potential of APC in severe inflammatory disorders.20,28
We propose a mechanism through which binary complexes of polyP with either HMGB1 or H4 initiates proinflammatory signaling in endothelial cells. The requirement for P2Y1 signaling at low polyP concentrations suggests the HMGB1/H4-loaded polyP binding to the P2Y1 receptor is associated with increased affinity of nuclear cytokines for their specific cell surface receptor, RAGE, through a bridging mechanism (Figure 7). We propose polyP bridging stabilizes dimeric or oligomeric forms of RAGE, facilitating the receptor clustering required for RAGE signaling.7 RAGE clustering brings cytoplasmic tails of the receptor into close proximity, enabling this domain to cooperatively interact with the adaptor molecule mDia1, which is responsible for initiating downstream signaling.7 PolyP-mediated bridging also increases ligand density on the cell surface, thereby amplifying the signal. Receptor stabilization by bridging can induce a positive feedback loop, thereby increasing expression of RAGE and further amplifying signaling. The observation that a near equimolar concentration of sRAGE effectively inhibits the signaling of HMGB1 in the absence of polyP-70, whereas a >100-fold excess of sRAGE is required to inhibit polyP-mediated signaling by HMGB1 is consistent with receptor bridging of polyP through P2Y1 playing a key role in clustering and amplification of RAGE signaling. We discovered this mechanism of polyP-mediated RAGE signaling occurs more efficiently with the longer chain polyP-700 that may be released during bacterial infection.14 Complement-mediated bacterial lysis may result in the release of longer chain polyP molecules that can bind many more nuclear cytokines, thereby dramatically amplifying proinflammatory responses through receptor bridging and clustering. It should be noted that DNA-bound nuclear cytokines have been reported to require TLR9 for eliciting effective proinflammatory signaling responses through RAGE in dendritic cells and B cells.29,30 Further studies are required to determine whether TLR9 makes a similar contribution to polyP-mediated nuclear cytokine signaling through RAGE in endothelial cells.
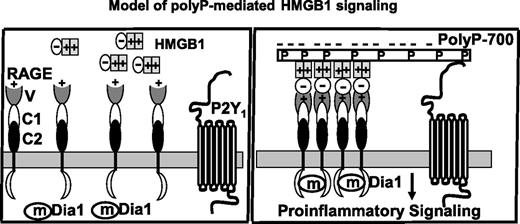
Hypothetical model of polyP-mediated proinflammatory nuclear cytokines (HMGB1 or H4) signaling. The 2 nuclear cytokines (only HMGB1 used in the graph) bind to the positively charged N-terminus variable domain (V) of RAGE receptor to elicit proinflammatory signaling responses in endothelial cells (or cells of the innate immune system). In the case of HMGB1, this can occur through the acidic C-terminus of the protein (shown in the model as a minus sign). However, relatively high concentrations of nuclear cytokines are required to initiate signaling because both HMGB1 and H4 are also highly positively charged. The interaction of the acidic polyP polymers with positively charged residues of either nuclear protein neutralizes the basic charges of these residues, thereby eliminating their repulsive interactions with the positively charged ligand-binding domain of RAGE (V) and enhancing their affinity for the receptor. Depending on its polymer size, polyP can bind to multiple nuclear cytokines (ie, polyP-700 shown in the model), thus simultaneously activating multiple receptors. Because polyP can also bind to P2Y1, this interaction is further enhanced by a bridging mechanism. The polyP-loaded ligand interaction with RAGE results in the clustering of the receptor and the P2Y1 bridging stabilizes oligomeric forms of RAGE, thus facilitating the cooperative interaction of the cytoplasmic tails of the receptor with the adaptor molecule mDia1, which is responsible for initiating downstream signaling. In the absence (or limiting concentration) of nuclear cytokines, polyP itself can also bind to either RAGE or P2Y1 separately or to both receptors by a bridging mechanism to initiate inflammatory signaling responses (not shown). V, C1, and C2 represent the N-terminal variable region followed by constant regions 1 and 2 of the extracellular domain of RAGE.
Hypothetical model of polyP-mediated proinflammatory nuclear cytokines (HMGB1 or H4) signaling. The 2 nuclear cytokines (only HMGB1 used in the graph) bind to the positively charged N-terminus variable domain (V) of RAGE receptor to elicit proinflammatory signaling responses in endothelial cells (or cells of the innate immune system). In the case of HMGB1, this can occur through the acidic C-terminus of the protein (shown in the model as a minus sign). However, relatively high concentrations of nuclear cytokines are required to initiate signaling because both HMGB1 and H4 are also highly positively charged. The interaction of the acidic polyP polymers with positively charged residues of either nuclear protein neutralizes the basic charges of these residues, thereby eliminating their repulsive interactions with the positively charged ligand-binding domain of RAGE (V) and enhancing their affinity for the receptor. Depending on its polymer size, polyP can bind to multiple nuclear cytokines (ie, polyP-700 shown in the model), thus simultaneously activating multiple receptors. Because polyP can also bind to P2Y1, this interaction is further enhanced by a bridging mechanism. The polyP-loaded ligand interaction with RAGE results in the clustering of the receptor and the P2Y1 bridging stabilizes oligomeric forms of RAGE, thus facilitating the cooperative interaction of the cytoplasmic tails of the receptor with the adaptor molecule mDia1, which is responsible for initiating downstream signaling. In the absence (or limiting concentration) of nuclear cytokines, polyP itself can also bind to either RAGE or P2Y1 separately or to both receptors by a bridging mechanism to initiate inflammatory signaling responses (not shown). V, C1, and C2 represent the N-terminal variable region followed by constant regions 1 and 2 of the extracellular domain of RAGE.
These findings may have major implications for the mechanism of polyP-mediated systemic inflammatory responses during severe sepsis and how a bacterial infection may overwhelm the immune system. Based on the data presented herein, we hypothesize that minute quantities of bacterial polyP can function as a sponge to concentrate highly proinflammatory nuclear cytokines (expected to be abundant in circulation during inflammation and coagulation) on cell surfaces of vascular and innate immune systems, thereby activating RAGE receptors by clustering and stabilizing them by bridging. This uncontrollable and devastating immune response may explain the characteristic high rate of rapid death in severe sepsis and difficulties in devising effective strategies to dampen such an uncontrolled inflammatory response. Our proposed mechanism points to novel therapeutic targets for the treatment of acute and chronic proinflammatory complications, including severe sepsis.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ms Audrey Rezaie for editorial work on the manuscript.
This work was supported by grants awarded by the National Institutes of Health, National Heart, Lung, and Blood Institute (HL 62565 and HL 101917) (A.R.R.).
Authorship
Contribution: P.D. and A.R.R. designed experiments and analyzed data; P.D., S.M.H., S.H.Q., C.M., and L.Y. performed research; J.C.E. contributed to writing the paper; and P.D. and A.R.R. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alireza R. Rezaie, Edward A. Doisy Department of Biochemistry and Molecular Biology, Saint Louis University School of Medicine, 1100 S Grand Blvd, St Louis, MO 63104; e-mail: rezaiear@slu.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal