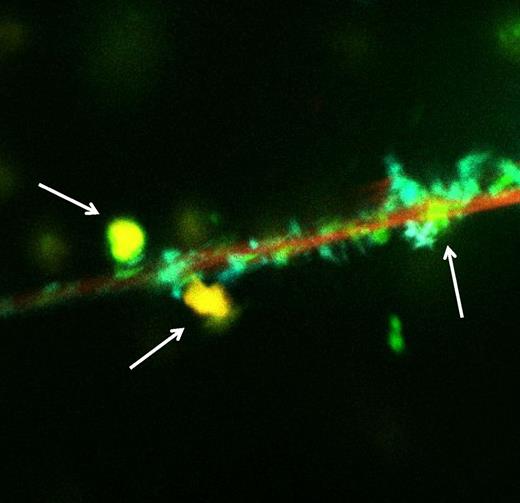
Fluor imaging analysis showing colocalization of histones, DNA, and nonanticoagulant heparin (yellow dots, arrows) on neutrophils (red surface). See the complete Figure 1 in the article by Wildhagen et al that begins on page 1098.
Fluor imaging analysis showing colocalization of histones, DNA, and nonanticoagulant heparin (yellow dots, arrows) on neutrophils (red surface). See the complete Figure 1 in the article by Wildhagen et al that begins on page 1098.
Activation of coagulation and inflammation are important and intertwined mechanisms in the pathogenesis of serious infection and sepsis.2 Proinflammatory cytokines and other mediators are capable of activating the coagulation system and down-regulating important physiological anticoagulant pathways. This may result in formation of microvascular thrombosis contributing to multiple organ dysfunction in patients with sepsis.3 Conversely, activated coagulation proteases may affect specific cellular receptors on inflammatory cells and endothelial cells and thereby modulate the inflammatory response. Hence, it is no surprise that in the quest to improve the outcome in sepsis, much attention has been directed to agents that attenuate the activation of both inflammation and coagulation. Heparin seems to be such an agent.
Heparin is a well-known anticoagulant to clinicians, and systematic reviews suggest that it may be beneficial in sepsis.4 However, the efficacy of heparin is clearly limited by the potential risk of major hemorrhage in critically ill patients. Interestingly, besides being an effective anticoagulant, there is mounting evidence that heparin is also a potent modulator of inflammation.4 Heparin inhibits the expression and function of adhesion molecules, such as P-selection and L-selectin. Moreover, heparin directly affects proinflammatory mediators, such as nuclear factor (NF)-κβ and cytokines, and attenuates endothelial cell dysfunction through the nitric oxide system.
The article of Wildhagen et al describes a novel and interesting immune-modulating mechanism of heparin.1 Heparin has a strong affinity for extracellular histones that result from cellular destruction during severe inflammation and that are robustly associated with endothelial dysfunction, organ failure, and death during sepsis5 (see figure). Interestingly, this property of heparin is independent of its anticoagulant function as a virtually nonanticoagulant heparin fraction had similar binding properties to histones. Binding of this nonanticoagulant heparin to histones strongly inhibited cytotoxic activity in vitro and translated to impaired inflammation and improved survival in animal models of systemic infection and inflammation.
This effect of heparin on histones fits well into the current ideas on the role of histones in the pathogenesis of sepsis and severe inflammation. Neutrophil extracellular traps (NETs) have recently been identified as important contributors to vascular thrombosis and inflammation.6 These NETs consist of extracellular DNA fibers that are present in inflammatory conditions as a result of programmed cell death of inflammatory cells and endothelial cells. NETs seem to be produced to allow inflammatory cells to trap and deactivate microorganisms in the extracellular environment by forming scaffolds of intact chromatin fibers with antimicrobial proteins. However, NETs may also induce endothelial cell death and detrimental inflammatory activity, an effect likely mediated by NET-associated proteases or cationic proteins, including histones.6,7 Importantly, it has been demonstrated that activated protein C is an significant inhibitor of histone-mediated detrimental effects in sepsis. Because there is ample interaction between heparin and activated protein C, it is likely that part of the heparin effect on histones is due to modulation of this activated protein C effect, which is in line with very recent observations in mice.8
The observation that this novel anti-inflammatory effect of heparin is independent of its anticoagulant activity may be relevant because the risk of hemorrhage is a major limitation of relatively high doses of heparin in patients with severe infection or sepsis who are already vulnerable to this complication due to low levels of platelets and coagulation factors. With the relatively simple technique as demonstrated in the paper by Wildhagen et al, it should be possible to produce heparin mixtures that are only partly anticoagulant but will maintain their full anti-inflammatory properties, providing an optimal therapeutic agent targeted at both coagulation and inflammation in severe sepsis and other types of systemic inflammation.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal