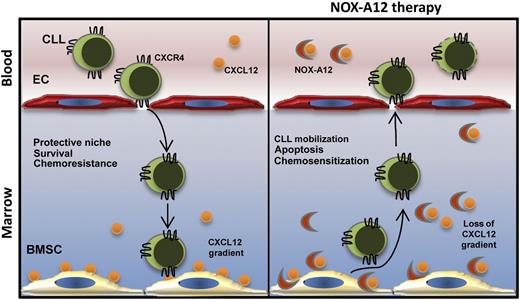
Schematic overview of NOX-A12 mechanism of action in CLL. NOX-A12 detaches CXCL12 from the surface of bone marrow stromal cells (BMSCs), thereby neutralizing the chemokine. As a consequence, NOX-A12 mobilizes CLL cells from their protective microenvironment, inducing apoptosis and chemosensitization on leukemic cells. EC, endothelial cell.
Schematic overview of NOX-A12 mechanism of action in CLL. NOX-A12 detaches CXCL12 from the surface of bone marrow stromal cells (BMSCs), thereby neutralizing the chemokine. As a consequence, NOX-A12 mobilizes CLL cells from their protective microenvironment, inducing apoptosis and chemosensitization on leukemic cells. EC, endothelial cell.
Lymphocytes continually recirculate from blood to tissues and back to the bloodstream again. Trafficking is mediated by transient interactions with endothelium through adhesion molecules and chemokines that trigger integrin activation, thus inducing firm adhesion and transendothelial migration into tissues where stromal cells guide lymphocyte homing and retention.2 In CLL, trafficking and homing into the lymph nodes and bone marrow is an essential part of the disease pathogenesis and progression. CLL dissemination inside tissue microenvironments is actively coordinated by crosstalk between leukemic cells and stroma. Attraction of CLL cells is mediated by gradients of chemokines through activation of corresponding chemokine receptors. In the marrow, CXCL12 (formerly called stromal cell-derived factor-1/SDF-1) secreted by stromal cells, guides leukemic cells inside the supportive microenvironments through activation of the corresponding receptor CXCR4 (CD184) expressed on the surface of CLL cells (see figure). In addition, CXCL12 not only mediates CLL cell chemotaxis, actin polymerization, and migration beneath and underneath CXCL12-secreting stromal cells but also protects CLL cells from spontaneous and drug-induced apoptosis.3
The necessity of CLL cells to localize into tissues and to establish an interactive network of cellular contacts with microenvironmental elements of immune and stromal systems may represent the Achilles heel of leukemic cells. How can we interfere with CXCL12/CXCR4 signaling, thus removing CLL cells from the nurturing and protective microenvironment and making them more vulnerable to conventional therapy? Different strategies have been developed to inhibit this supporting signal, mainly focusing on the blockade of CXCR4 either with small-molecule CXCX4 antagonists such as plerixafor or with anti-CXCR4 monoclonal antibodies.4 NOX-A12 is a novel inhibitor of CXCL12 and belongs to the substance class of PEGylated Spiegelmers. PEGylated Spiegelmers are single-stranded RNA oligonucleotides (aptamers) in an L-configuration (ie, the mirror image of naturally occurring RNA) containing a branched 40 kDa polyethylene glycol moiety. They are highly resistant to nuclease degradation, immunologically passive, and capable of tightly and specifically targeting molecules to inhibit their function.5
The article by Hoellenriegel et al1 describes a novel mechanism of action exerted by NOX-A12 in CLL. The investigators inspected the effect of NOX-A12 on CXCL12-related CLL cell functions and drug sensitivity. CLL cells were allowed to migrate toward CXCL12 in the presence or absence of NOX-A12. They demonstrated that NOX-A12 reduces CXCL12-induced CLL chemotaxis already at the lower concentration of 3 nM. To further support this observation, 2 lymphoid cell lines (Jurkat and Nalm-6) were tested in migration assays toward CXCL12, revealing an inhibition of chemotaxis in the presence of NOX-A12. Surprisingly, they also found that NOX-A12 promotes migration of CLL cells (and also of the lymphoid cell lines) beneath BMSCs (ie, pseudoemperipolesis [PEP]) by using murine stromal 9-15C, TSt-4, and MS-5 cell lines. Conversely, ADM3100 (CXCR4 antagonist) mediated a robust inhibition of PEP. To explain these apparently conflicting results, they hypothesized that the increase in PEP of CLL cells might be the result of NOX-A12–induced CXCL12 removal from stromal cell surface, thus establishing a positive gradient toward high CXCL12 concentrations underneath the stromal layer. CXCL12 is bound to glycosaminoglycans (GAGs) (eg, heparin) on the cell surface, causing CXCL12 surface retention and exposure for activation of CXCR4.6 Hoellenriegel et al clearly demonstrated that NOX-A12 leads to rapid release of CXCL12 from stromal cell surface–bound GAGs, thereby neutralizing the chemokine (see figure). Three main observations corroborate this NOX-A12 mode of action: (1) CXCL12 levels in the supernatant of BMSCs suddenly increase after NOX-A12 treatment, although treatment does not affect intracellular CXCL12 content; (2) recombinant CXCL12 binds to the surface of BMSCs, whose endogenous CXCL12 was stripped from the cell surface upon NOX-A12 treatment; and (3) NOX-A12 detaches CXCL12 bound to immobilized heparin. Last, Hoellenriegel et al focused on CLL spontaneous and drug-induced apoptosis in the CXCL12-depleted BMSC model upon NOX-A12 treatment. A slight but significant decrease of viability and, more importantly, the reduction of BMSC-mediated chemoprotection (bendamustine and fludarabine) were detected in CLL cells cultured in contact with NOX-A12–treated stromal cells, suggesting an effect of chemosensitization. Further points to be addressed could concern the effect of NOX-A12 on CXCR4 downstream signaling events and also on primary BMSCs from CLL patients or other microenvironmental supportive elements, such as nurse-like cells, that also express CXCL12 and attract CLL cells by elaboration of this chemokine. In addition, the in vitro data might be corroborated by in vivo studies in mouse models.
NOX-A12 represents the first CXCL12-specific Spiegelmer, with unique properties including high binding specificity, low immunogenicity, and structural stability. In a clinical phase 1 trial in healthy volunteers, NOX-A12 treatment mobilized white blood cells, in particular lymphocytes, neutrophils, and monocytes (4.4-, 2.5- and 3.7-fold, respectively, as compared with basal levels), and CD34+ cells into peripheral blood. NOX-A12 has proved to be safe and well tolerated in this study.7 A similar efficacy in leukocyte mobilization characterizes NOX-A12 and plerixafor. Nevertheless, NOX-A12 seems to have a more pronounced taste for lymphocytes and to mediate a longer-term mobilization of leukemic cells than plerixafor.7 A treatment concept that would first remove CLL cells from their protective microenvironment by NOX-A12 and then target more vulnerable CLL cells with cytotoxic agents such as fludarabine and bendamustine might improve the efficacy of current therapeutic strategies. In view of this, NOX-A12 is currently being evaluated in an ongoing phase 2a clinical trial in combination with bendamustine/rituximab (BR). Preliminary results of the pilot group (n = 10) who received single intravenous doses of 1, 2, or 4 mg/kg body weight 2 weeks prior to 6 cycles of combined treatment of NOX-A12 with BR showed effective and long-lasting mobilization of CLL cells into the peripheral blood, 100% overall response rate with 22% complete remission, and no additional toxicity.8
The preclinical study of Hoellenriegel et al provides novel insights into the NOX-A12 mode of action, paving the way toward a novel paradigm of therapy that moves from cancer cells to microenvironmental elements as the primary target of treatments. Results from correlative studies in the ongoing clinical trial of NOX-A12 in CLL will provide further clarification into the mechanism of action of NOX-A12 on CLL cell distribution and chemosensitization and, above all, into safety and efficacy of the first CXCL12-specific Spiegelmer.
Conflict-of-interest disclosure: The authors declare no competing financial interests.