Key Points
In P falciparum–infected anemic children, immature gametocytes are more prevalent and abundant in bone marrow than in peripheral blood.
P falciparum–infected anemic children are gametocyte carriers that can potentially contribute to malaria transmission.
Plasmodium falciparum immature gametocytes are not observed in peripheral blood. However, gametocyte stages in organs such as bone marrow have never been assessed by molecular techniques, which are more sensitive than optical microscopy. We quantified P falciparum sexual stages in bone marrow (n = 174) and peripheral blood (n = 70) of Mozambican anemic children by quantitative polymerase chain reaction targeting transcripts specific for early (PF14_0748; PHISTa), intermediate (PF13_0247; Pfs48/45), and mature (PF10_0303; Pfs25) gametocytes. Among children positive for the P falciparum housekeeping gene (PF08_0085; ubiquitin-conjugating enzyme gene) in bone marrow (n = 136) and peripheral blood (n = 25), prevalence of immature gametocytes was higher in bone marrow than peripheral blood (early: 95% vs 20%, P < .001; intermediate: 80% vs 16%; P < .001), as were transcript levels (P < .001 for both stages). In contrast, mature gametocytes were more prevalent (100% vs 51%, P < .001) and abundant (P < .001) in peripheral blood than in the bone marrow. Severe anemia (3.57, 95% confidence interval 1.49-8.53) and dyserythropoiesis (6.21, 95% confidence interval 2.24-17.25) were independently associated with a higher prevalence of mature gametocytes in bone marrow. Our results highlight the high prevalence and abundance of early sexual stages in bone marrow, as well as the relationship between hematological disturbances and gametocyte development in this tissue.
Introduction
Cytoadhesive interactions with host receptors mediate the sequestration of Plasmodium falciparum mature asexual parasites in different organs and tissues, a phenomenon that is suggested to reduce destruction of infected erythrocytes in the spleen and eventually lead to severe malaria.1 A similar phenomenon has been hypothesized for the immature stages of gametocytes that, after maturation, are responsible for Plasmodium transmission from humans to the mosquito.2 However, the organs where gametocytes develop and the mechanisms underlying this process are not well defined.
A small proportion of P falciparum parasites divert from the asexual erythrocytic cycle and develop into gametocytes through a maturation process classically divided into 5 morphological stages (I-V).3,4 Factors contributing to the genesis and development of gametocytes in vivo are largely unknown. Because only gametocytes at the mature stage V are microscopically detectable in peripheral blood,5 it has been suggested that developing stages may be retained in internal organs. Evidence for such enrichment is scarce, with several case reports showing an abundance of immature gametocytes in the spleen and bone marrow.5,,-8 However, these studies are of limited scale and used standard light microscopy as the only method for gametocyte detection and stage differentiation; standard light microscopy is known to have low sensitivity compared with molecular tools9,10 and to miss a substantial proportion of P falciparum infections in surveys of endemic populations.11,-13 The information on gametocyte stages detected by molecular techniques such as polymerase chain reaction (PCR) is scarce for P falciparum infections in peripheral blood and absent for infections in the bone marrow. Such a lack of data limits the accurate assessment of malaria transmission at a community level, which is critical to enabling rational development of a transmission-blocking vaccine14 and to support elimination and eradication of P falciparum and P vivax.15
A better understanding of the dynamics between the multiplication of P falciparum, gametocytogenesis, and malaria transmission is essential to enabling development of new tools to disrupt malaria transmission.15 To expand investigation beyond case reports and autopsy studies and overcome limitations in the sensitivity and gametocyte stage differentiation of optical microscopy, we used a stage-specific quantitative reverse-transcriptase PCR (qPCR) to compare the prevalence and levels of gametocytes in 174 bone marrow samples and 70 matched peripheral blood samples from Mozambican anemic children, and identified factors associated with the development of sexual stages.
Methods
Study site, participants, and sample collection
The study was carried out at the Centro de Investigação em Saúde de Manhiça in the Manhiça District of southern Mozambique.16 Malaria transmission, mainly from P falciparum (95%), is of moderate and perennial intensity with marked seasonality. To assess dynamics of P falciparum gametocyte stages in bone marrow and peripheral blood of anemic children, 174 1- to 59-month-old children admitted to the Manhiça District Hospital between October 2008 and August 2010 with hemoglobin <11 g/dL by the HemoCue system (HemoCue HB 201+; Änghelom, Sweden) and no history of blood transfusion in the preceding 4 weeks who had undergone bone marrow aspiration were recruited as participants.
A complete clinical examination of the 174 children recruited for the study was performed, and the information was recorded onto standardized questionnaires together with demographic data. P falciparum was detected by microscopy in peripheral blood films following standard, quality-controlled procedures.17 Four milliliters of venous blood were collected by venipuncture into a heparinized Vacutainer from a subset of children (n = 70). Three to 4 mL of bone marrow were aspirated from the anterosuperior iliac crest or the tibia of the 174 children under conscious sedation with parenteral atropine, ketamine, and diazepam.18,19 The first drops of the aspirate were used to prepare smears and the rest of the sample was collected into an EDTA-coated Vacutainer. Bone marrow aspirates were not performed in children <3 months of age or with medical counterindications such as severe respiratory distress, recent history of seizures, suspected intracranial hypertension, or any other risk identified by the responsible pediatrician. Resuscitation equipment was always available during the procedure.
The study protocol was approved by the National Mozambican Ethics Committee and the Hospital Clínic of Barcelona Ethics Review Committee. The parents/guardians of all children included in the study provided written informed consent after being informed of the goals, benefits, and risks of the procedures and were offered no financial or material inducements for participation. This study was conducted in accordance with the Declaration of Helsinki. All children received treatment according to national guidelines.
Bone marrow smear examination
Bone marrow smears were fixed and stained with May-Grünwald eosin methylene blue solution and 10% Giemsa for cytological and parasitological examination. Smear cytology was evaluated by an experienced hematologist who was blinded to clinical and laboratory details. Dyserythropoiesis was successfully assessed in 136 of the bone marrow smears and defined as the presence of erythroblasts with binuclearity and trinuclearity, internuclear chromatin bridges, nuclear pyknosis, or any other chromatin alterations.20 Bone marrow smears were considered microscopically negative for P falciparum if no parasites were detected in 100 fields at ×100 magnification. Hemozoin, identified as coarse brown granular material with birefringence under polarized light,21 was assessed in bone marrow cellular aggregates observed in 132 of the smears. Stage I gametocytes were defined as round parasites, sometimes with slightly pointed ends, and hemozoin located in elongated granules; stage II as oat grain-shaped parasites with pointed ends and scattered granules of hemozoin; stage III as elongated “D”-shaped parasites; stage IV as symmetrical, elongated, spindle-shaped parasites with pointed ends; and stage V as sausage-shaped parasites with rounded ends.3,22,23
CRP and EPO measurements
Peripheral blood was centrifuged and plasma samples were used to quantify C-reactive protein (CRP) and erythropoietin (EPO) levels, using an ADVIA 2400 automated analyzer (Siemens Healthcare, Barcelona, Spain) and the Quantikine human Erythropoietin kit (R&D Systems, Minneapolis, MN), respectively.
RNA extraction and cDNA synthesis
Two milliliters of bone marrow aspirate were transferred to a Paxgene Bone Marrow RNA Tube (QIAGEN) and 100 µL of peripheral red blood cells to 1 mL of Trizol (Invitrogen) for RNA stabilization. Samples were stored at −80°C until RNA preparation using the Paxgene Bone Marrow RNA Kit (QIAGEN) and the PureLink RNA Mini kit (Invitrogen) for bone marrow and peripheral blood samples, respectively. Quantity and purity of RNA were assessed with a Bioanalyser 2100 (Agilent Technologies) or a Nanodrop spectrophotometer (Thermo Scientific). RNA was treated with DNAse using the Turbo DNA-free kit (Invitrogen). Contamination with genomic parasite DNA was assessed by a TaqMan qPCR specific for the P falciparum 18S ribosomal gene 24,25 ; positive samples were retreated with DNAse and retested for lack of genomic DNA. RNA (2 µg) was used for complementary DNA (cDNA) conversion with the SuperScript VILO cDNA Synthesis Kit (Invitrogen) using a 2-hour incubation step at 42°C to maximize yields.
qPCR of gametocyte maturation stages
Early, intermediate, and mature gametocyte transcripts were detected using primers for 3 gametocyte markers selected from published microarray expression data for their tight windows of high expression during a certain period of gametocyte development.26,-28 These primers have been previously developed and validated in a study on autopsy tissue sections by Joice et al (R.J., Sandra K. Nilsson, Jacqui Montgomery, Selasi Dankwa, Elizabeth Egan, Belinda Morahan, Karl B. Seydel, Lucia Bertuccini, Pietro Alano, Kim C. Williamson, Manoj T. Duraisingh, Terrie E. Taylor, Danny A. Milner, M.M., manuscript submitted September 2013): PHISTa gene (PF14_0748; peak expression in early gametocytes, stages I-II; forward (Fw): ATT CAA GGG TAG TTC CTA GAG CAG TGT GG; reverse (Rv): AGC ACT CGT AAT TCT AAC ACT GGG) as a marker of young gametocytes; transmission-blocking antigen precursor Pfs48/45 (PF13_0247; peak in intermediate gametocytes, stages III-IV; Fw: GTA AGC CTA GCT CTT TGA ATA GTG A; Rv: GAC CTA CGT TCA CGC ATA TCT GGC T) for intermediate gametocytes; and ookinete surface antigen precursor Pfs25 (PF10_0303; peak in mature gametocytes, stage V; Fw: GGA AAT CCC GTT TCA TAC GCT TGT; Rv: TCT TGT ACA TTG GGA ACT TTG CCT) for late developing and mature gametocytes. Final reaction volumes of 20 µL included 4 µL of cDNA (∼8 ng/µL) and 10 µL of iQ SYBR Green Master Mix (BioRad). qPCRs were performed in a Viaa7 analyzer (Applied Biosystems) with 10 minutes at 95°C (1 cycle), 30 seconds at 95°C, and 1 minute at 58°C (40 cycles). Each sample was analyzed in triplicate alongside a dilution series of cDNA from messenger RNA targets cloned into pGEM plasmids for absolute quantification of transcript copy numbers. Positive transcription was defined as a cycle threshold value within the standard curve’s cycle threshold range. To normalize for the level of parasitemia and amount of cDNA loaded in each reaction, relative transcript levels were calculated as the ratio between copy numbers of gametocyte transcripts and the P falciparum ubiquitin-conjugating enzyme (PF08_0085) transcript (housekeeping [HK] gene). This HK gene was chosen for being highly constitutively expressed across patients and parasite life-cycle stages,29,,-32 and was also quantified by qPCR (Fw: GGT GTT AGT GGC TCA CCA ATA GGA; Rv: GTA CCA CCT TCC CAT GGA GTA TCA), as described previously.33
Definitions and statistical methods
Fever was defined as axillary temperature ≥37.5°C, moderate anemia as hemoglobin concentration between 11 and 7 g/dL, and severe anemia as hemoglobin concentration <7 g/dL. The high and low EPO, CRP, and age categories were defined based on their median values from the 174 children included in the study. Asexual submicroscopic parasitemias, defined as a parasite-negative result by microscopy but positive by qPCR for the P falciparum HK gene, were assigned a parasite density of 0.1 parasites per microliter of blood. Categorical and continuous variables were compared between groups using the Fisher exact test and Mann-Whitney U test, respectively. McNemar and Wilcoxon signed-rank tests were used for comparisons between paired samples. The correlations between relative transcript copy numbers and parasite density were assessed by Spearman test. Associations of the prevalence of gametocyte stages in the bone marrow and of log-transformed relative transcript copy numbers with demographic and clinical parameters were assessed by Fisher test and Student t test, respectively. Backward stepwise regression models were performed including variables significantly associated in the univariate analysis. Multiple imputation analysis was performed on the missing values (range 1.83%-25.71%) using the Amelia II software, version 1.6.3. Regression was carried out on each of the 10 imputed datasets and the results were combined. Statistical analyses were performed using STATA.12 (STATA Corporation, College Station, TX). All P values are 2-sided and were considered significant when <.05.
Results
Characteristics of the study participants
A total of 174 anemic children who had undergone bone marrow aspiration were included in this study (Table 1). The median age of participants was 20 months (interquartile range [IQR] 12, 30), and 55% (95/174) were male. The median hemoglobin concentration was 6.9 (IQR 5.4, 9.6) g/dL, with 50% (88/174) of the children being severely anemic (hemoglobin <7 g/dL) and 57% (99/174) presenting with a fever at sample collection. Hemozoin was observed in 54% (71/132) of the bone marrow samples and signs of dyserythropoiesis in 67% (91/136), with nuclear pyknosis followed by irregular nuclei as the most commonly detected anomalies. The prevalence of dyserythropoiesis was similar in children with and without fever (36 of 57 [63%] vs 55 of 91 [60%], P = .464). Children with severe anemia were older and had a higher prevalence of hemozoin and asexual P falciparum infection detected by microscopy in the bone marrow, as well as in peripheral blood, compared with nonseverely anemic children (Table 1). Seventy-eight percent (136/174) of the bone marrow samples and 36% (25/70) of the peripheral blood samples were positive for the P falciparum HK gene by qPCR. Sixteen (23%) of the 70 children with data from both bone marrow and peripheral blood samples were positive for the P falciparum HK gene in both compartments.
Baseline characteristics of children included in the study
| . | Severe anemia . | . | |
|---|---|---|---|
| . | No (n = 86) . | Yes (n = 88) . | P . |
| Age (months) (median, IQR) | 16 (10, 28) | 23.5 (16, 33) | .002 |
| Male (n, %) | 47 (55) | 48 (55) | 1.000 |
| Hemoglobin (g/dL) (median, IQR) | 9.65 (9.2, 10.2) | 5.4 (4.3, 6.15) | <.001 |
| Fever (n, %) | 48 (56) | 51 (58) | .878 |
| Hemozoin (n, %)* | 18 (27) | 53 (81) | <.001 |
| Dyserythropoiesis (n, %)† | 39 (59) | 52 (74) | .070 |
| Asexual parasitemia (microscopy) | |||
| Bone marrow (n, %)‡ | 18 (22) | 55 (66) | <.001 |
| Peripheral blood (n, %) | 20 (23) | 55 (62) | <.001 |
| . | Severe anemia . | . | |
|---|---|---|---|
| . | No (n = 86) . | Yes (n = 88) . | P . |
| Age (months) (median, IQR) | 16 (10, 28) | 23.5 (16, 33) | .002 |
| Male (n, %) | 47 (55) | 48 (55) | 1.000 |
| Hemoglobin (g/dL) (median, IQR) | 9.65 (9.2, 10.2) | 5.4 (4.3, 6.15) | <.001 |
| Fever (n, %) | 48 (56) | 51 (58) | .878 |
| Hemozoin (n, %)* | 18 (27) | 53 (81) | <.001 |
| Dyserythropoiesis (n, %)† | 39 (59) | 52 (74) | .070 |
| Asexual parasitemia (microscopy) | |||
| Bone marrow (n, %)‡ | 18 (22) | 55 (66) | <.001 |
| Peripheral blood (n, %) | 20 (23) | 55 (62) | <.001 |
IQR, interquartile range. P values are from Fisher (categorical variables) and Mann-Whitney tests (continuous variables).
42 missing.
38 missing.
11 missing.
P falciparum gametocyte stages in bone marrow and peripheral blood
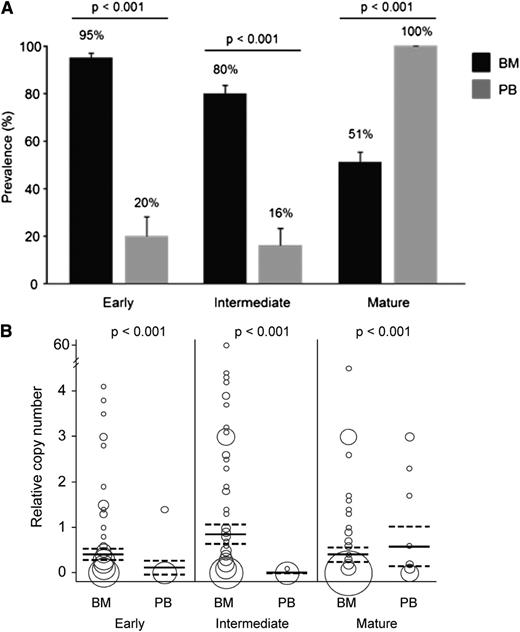
Sexual P falciparum parasites were detected by microscopy in the peripheral blood of 5% (9/174) of the children and in the bone marrow of 28% (46/162) (Table 2). Prevalence and relative transcript copy numbers of qPCR-detected gametocyte stages in bone marrow and peripheral blood are shown in Table 2 and Figure 1. Among the 136 bone marrow samples and 25 peripheral blood samples that were positive for P falciparum based on qPCR detection of the HK gene, 133 (98%) and 25 (100%), respectively, were positive for at least 1 gametocyte stage marker. Of these gametocyte qPCR-positive samples, microscopy data were available for 124 bone marrow samples with 27 (22%) gametocyte-positive (Figure 2) and for 25 peripheral blood samples with 3 (12%) gametocyte-positive. Prevalence of early and intermediate gametocyte stages in bone marrow (n = 136) were 4.8 and 5 times higher, respectively, compared with peripheral blood (n = 25), whereas mature gametocytes were twice as prevalent in peripheral blood as in bone marrow (P < .001 in the 3 comparisons; Figure 1A). Similarly, relative transcript levels of early and intermediate gametocyte markers were higher in bone marrow compared with peripheral blood, and mature gametocyte transcripts were higher in peripheral blood than in bone marrow (P < .001 in the 3 comparisons; Figure 1B). In the paired analysis including the 16 children positive by qPCR for P falciparum both in bone marrow and peripheral blood, early and intermediate stages were found to be significantly more prevalent and more highly expressed in bone marrow compared with peripheral blood (Table 3).
Prevalence of gametocyte-positive samples determined by microscopy and qPCR
| . | Gametocytes, n (%) . | |||
|---|---|---|---|---|
| . | Microscopy . | qPCR . | ||
| . | Peripheral blood . | Bone marrow* . | Peripheral blood . | Bone marrow . |
| Positive | 9 (5.2) | 46 (28.4) | 25 (14.4)† | 133 (76.4)† |
| Negative | 165 (94.8) | 116 (71.6) | 149 (85.6) | 41 (23.6) |
| . | Gametocytes, n (%) . | |||
|---|---|---|---|---|
| . | Microscopy . | qPCR . | ||
| . | Peripheral blood . | Bone marrow* . | Peripheral blood . | Bone marrow . |
| Positive | 9 (5.2) | 46 (28.4) | 25 (14.4)† | 133 (76.4)† |
| Negative | 165 (94.8) | 116 (71.6) | 149 (85.6) | 41 (23.6) |
12 samples not determined.
Samples positive for at least 1 of the 3 gametocyte-stage markers.
P falciparum gametocyte stages in bone marrow (BM) and peripheral blood (PB) detected by qPCR. (A) Prevalence and standard error of early, intermediate, and mature gametocyte stages. (B) Relative transcript copy numbers expressed as the ratio between copy numbers of gametocyte transcripts and P falciparum ubiquitin-conjugating enzyme transcript. The area of the circle is proportional to the number of observations, and mean copy number and 95% confidence intervals are indicated by horizontal lines. Samples positive for the housekeeping gene are included in the analysis: 136 BM and 25 PB samples.
P falciparum gametocyte stages in bone marrow (BM) and peripheral blood (PB) detected by qPCR. (A) Prevalence and standard error of early, intermediate, and mature gametocyte stages. (B) Relative transcript copy numbers expressed as the ratio between copy numbers of gametocyte transcripts and P falciparum ubiquitin-conjugating enzyme transcript. The area of the circle is proportional to the number of observations, and mean copy number and 95% confidence intervals are indicated by horizontal lines. Samples positive for the housekeeping gene are included in the analysis: 136 BM and 25 PB samples.
Prevalence of P falciparum gametocyte stages in gametocyte-positive bone marrows as detected by microscopy. Prevalence and standard error of the different gametocyte stages in bone marrow samples that are gametocyte-positive by microscopy (n = 27).
Prevalence of P falciparum gametocyte stages in gametocyte-positive bone marrows as detected by microscopy. Prevalence and standard error of the different gametocyte stages in bone marrow samples that are gametocyte-positive by microscopy (n = 27).
Paired analyses comparing the prevalence and transcript levels of gametocyte stage markers between bone marrow and peripheral blood
| . | Prevalence . | Relative transcript copy number* . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | Peripheral blood . | Bone marrow–Peripheral blood . | ||||||
| Bone marrow . | Positive . | Negative . | P† . | n > 0‡ . | n < 0‡ . | Median§ . | Interquartile range . | P¶ . |
| Early | ||||||||
| Positive | 3 | 13 | <.001 | 15 | 1 | 0.098 | 0.043, 0.295 | .004 |
| Negative | 0 | 0 | ||||||
| Intermediate | ||||||||
| Positive | 3 | 13 | <.001 | 16 | 0 | 0.210 | 0.034, 1.696 | <.001 |
| Negative | 0 | 0 | ||||||
| Mature | ||||||||
| Positive | 15 | 0 | .032 | 10 | 6 | 0.110 | −0.047, 1.539 | .148 |
| Negative | 1 | 0 | ||||||
| . | Prevalence . | Relative transcript copy number* . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | Peripheral blood . | Bone marrow–Peripheral blood . | ||||||
| Bone marrow . | Positive . | Negative . | P† . | n > 0‡ . | n < 0‡ . | Median§ . | Interquartile range . | P¶ . |
| Early | ||||||||
| Positive | 3 | 13 | <.001 | 15 | 1 | 0.098 | 0.043, 0.295 | .004 |
| Negative | 0 | 0 | ||||||
| Intermediate | ||||||||
| Positive | 3 | 13 | <.001 | 16 | 0 | 0.210 | 0.034, 1.696 | <.001 |
| Negative | 0 | 0 | ||||||
| Mature | ||||||||
| Positive | 15 | 0 | .032 | 10 | 6 | 0.110 | −0.047, 1.539 | .148 |
| Negative | 1 | 0 | ||||||
n = 16 paired samples.
Ratio between copy numbers of gametocyte transcripts and P falciparum ubiquitin-conjugating enzyme transcript.
Prevalences were compared using the McNemar test.
Number of pairs showing a higher (n > 0) or lower (n < 0) copy number of transcripts in bone marrow compared with peripheral blood.
Median of the difference.
Relative transcript copy numbers were compared using the Wilcoxon signed-rank test.
Parameters associated with gametocyte stages in bone marrow
The univariate analyses showed that the presence of microscopically detected parasites and/or hemozoin in bone marrow, age >20 months, high CRP and EPO levels, severe anemia, and dyserythropoiesis were significantly associated with an increased prevalence (Table 4) and relative transcript copy numbers (Table 5) of mature gametocytes in bone marrow. Moreover, relative transcript copy numbers of mature stages in bone marrow were positively correlated with parasite densities in peripheral blood (ρ = 0.301, P < .001). The backward stepwise regression analysis with imputed data showed independent positive associations between the prevalence of mature stages and severe anemia (P = .004), dyserythropoiesis (P < .001), as well as the presence of a microscopic infection in the bone marrow (P = .004) (Table 6). Moreover, relative transcript copy numbers of mature gametocytes were positively associated with severe anemia (P < .001) and negatively with fever (P = .003) (Table 6). Among the 25 children with qPCR-detected P falciparum infection in peripheral blood, relative transcript copy numbers of mature stages in peripheral blood were lower in severely anemic children (median: 0.086, IQR 0.014, 1.657) compared with those with moderate anemia (0.011, IQR 0.003, 0.671; P = .052).
Prevalence of early, intermediate, and mature gametocytes in bone marrow according to demographic and clinical parameters
| . | Early . | Intermediate . | Mature . | |||
|---|---|---|---|---|---|---|
| . | n (%) . | P . | n (%) . | P . | n (%) . | P . |
| Age (months) | ||||||
| ≤20 (n = 80) | 76 (95) | 1.000 | 61 (76) | .196 | 35 (44) | .037 |
| >20 (n = 56) | 53 (95) | 48 (86) | 35 (63) | |||
| Sex | ||||||
| Male (n = 77) | 73 (95) | 1.000 | 61 (79) | .830 | 39 (51) | .864 |
| Female (n = 59) | 56 (95) | 48 (81) | 31 (53) | |||
| Fever | ||||||
| No (n = 57) | 52 (91) | .130 | 42 (74) | .130 | 26 (46) | .298 |
| Yes (n = 79) | 77 (97) | 67 (85) | 44 (56) | |||
| CRP* | ||||||
| Low (n = 68) | 64 (94) | .682 | 51 (75) | .080 | 26 (38) | .003 |
| High (n = 63) | 61 (97) | 55 (87) | 41 (65) | |||
| Severe anemia | ||||||
| No (n = 74) | 71 (96) | .702 | 59 (80) | 1.000 | 24 (32) | <.001 |
| Yes (n = 62) | 58 (94) | 50 (81) | 46 (74) | |||
| EPO† | ||||||
| Low (n = 73) | 70 (96) | 1.000 | 60 (82) | .824 | 31 (42) | .024 |
| High (n = 59) | 56 (95) | 47 (80) | 37 (63) | |||
| Asexual parasitemia (BM)‡ | ||||||
| Submic (n = 81) | 76 (94) | 1.000 | 62 (77) | .173 | 29 (36) | <.001 |
| Patent (n = 46) | 44 (96) | 40 (87) | 35 (76) | |||
| Hemozoin | ||||||
| No (n = 56) | 52 (93) | .686 | 43 (77) | .472 | 16 (29) | <.001 |
| Yes (n = 47) | 45 (96) | 39 (83) | 33 (70) | |||
| Dyserythropoiesis | ||||||
| No (n = 33) | 30 (91) | .373 | 25 (76) | .422 | 7 (21) | <.001 |
| Yes (n = 73) | 70 (96) | 61 (84) | 45 (62) | |||
| . | Early . | Intermediate . | Mature . | |||
|---|---|---|---|---|---|---|
| . | n (%) . | P . | n (%) . | P . | n (%) . | P . |
| Age (months) | ||||||
| ≤20 (n = 80) | 76 (95) | 1.000 | 61 (76) | .196 | 35 (44) | .037 |
| >20 (n = 56) | 53 (95) | 48 (86) | 35 (63) | |||
| Sex | ||||||
| Male (n = 77) | 73 (95) | 1.000 | 61 (79) | .830 | 39 (51) | .864 |
| Female (n = 59) | 56 (95) | 48 (81) | 31 (53) | |||
| Fever | ||||||
| No (n = 57) | 52 (91) | .130 | 42 (74) | .130 | 26 (46) | .298 |
| Yes (n = 79) | 77 (97) | 67 (85) | 44 (56) | |||
| CRP* | ||||||
| Low (n = 68) | 64 (94) | .682 | 51 (75) | .080 | 26 (38) | .003 |
| High (n = 63) | 61 (97) | 55 (87) | 41 (65) | |||
| Severe anemia | ||||||
| No (n = 74) | 71 (96) | .702 | 59 (80) | 1.000 | 24 (32) | <.001 |
| Yes (n = 62) | 58 (94) | 50 (81) | 46 (74) | |||
| EPO† | ||||||
| Low (n = 73) | 70 (96) | 1.000 | 60 (82) | .824 | 31 (42) | .024 |
| High (n = 59) | 56 (95) | 47 (80) | 37 (63) | |||
| Asexual parasitemia (BM)‡ | ||||||
| Submic (n = 81) | 76 (94) | 1.000 | 62 (77) | .173 | 29 (36) | <.001 |
| Patent (n = 46) | 44 (96) | 40 (87) | 35 (76) | |||
| Hemozoin | ||||||
| No (n = 56) | 52 (93) | .686 | 43 (77) | .472 | 16 (29) | <.001 |
| Yes (n = 47) | 45 (96) | 39 (83) | 33 (70) | |||
| Dyserythropoiesis | ||||||
| No (n = 33) | 30 (91) | .373 | 25 (76) | .422 | 7 (21) | <.001 |
| Yes (n = 73) | 70 (96) | 61 (84) | 45 (62) | |||
P values are from Fisher test.
Submic, submicroscopic infections.
Median: 7.44 mg/dL.
Median: 88.65 U/L.
Submicroscopic if parasites were detected only by qPCR.
Relative transcript levels of early, intermediate, and mature gametocyte stage markers in bone marrow positive for the different gametocyte stages according to demographic and clinical parameters
| . | . | Relative transcript levels (×100)* . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Early . | . | Intermediate . | . | Mature . | |||
| . | n . | GM (95%CI) . | P . | n . | GM (95%CI) . | P . | n . | GM (95%CI) . | P . |
| Age (months) | |||||||||
| ≤20 | 76 | 17.2 (12.0, 24.9) | .296 | 61 | 40.8 (23.2, 72.0) | .314 | 35 | 8.5 (3.2, 22.7) | .200 |
| >20 | 53 | 12.8 (8.2, 19.8) | 48 | 26.3 (13.4, 51.4) | 35 | 20.3 (7.8, 52.6) | |||
| Sex | |||||||||
| Male | 73 | 14.9 (10.2, 21.9) | .871 | 61 | 29.9 (16.7, 53.3) | .541 | 39 | 13.6 (5.7, 32.4) | .90 |
| Female | 56 | 15.7 (10.3, 23.7) | 48 | 39.1 (20.2, 75.5) | 31 | 12.5 (4.1, 38.5) | |||
| Fever | |||||||||
| No | 52 | 21.0 (13.6, 32.4) | .061 | 42 | 48.0 (25.7, 89.4) | .195 | 26 | 40.6 (13.2, 125.1) | .009 |
| Yes | 77 | 12.3 (8.5, 17.7) | 67 | 26.9 (15.0, 48.3) | 44 | 6.7 (3.0, 15.1) | |||
| CRP† | |||||||||
| Low | 64 | 14.1 (9.2, 21.6) | .880 | 51 | 31.3 (15.9, 61.5) | .790 | 26 | 4.5 (1.5, 13.3) | .016 |
| High | 61 | 14.7 (10.2, 21.3) | 55 | 35.2 (19.6, 63.5) | 41 | 23.5 (10.1, 54.7) | |||
| Severe anemia | |||||||||
| No | 71 | 16.2 (11.2, 23.4) | .639 | 59 | 31.8 (16.8, 60.1) | .776 | 24 | 2.7 (0.9, 7.9) | <.001 |
| Yes | 58 | 14.2 (9.2, 21.9) | 50 | 36.0 (20.2, 64.2) | 46 | 30.1 (13.8, 65.4) | |||
| EPO‡ | |||||||||
| Low | 70 | 14.3 (9.9, 20.6) | .898 | 60 | 26.1 (14.2, 47.9) | .236 | 31 | 3.8 (1.4, 10.2) | .002 |
| High | 56 | 14.8 (9.6, 23.0) | 47 | 44.2 (23.5, 83.1) | 37 | 30.1 (12.8, 70.8) | |||
| Asexual parasitemia (BM)¶ | |||||||||
| Submic | 76 | 21.9 (15.9, 30.2) | .001 | 62 | 60.1 (37.9, 95.4) | <.001 | 29 | 9.7 (3.2, 29.3) | .386 |
| Patent | 44 | 8.4 (5.0, 14.1) | 40 | 10.7 (4.9, 23.3) | 35 | 17.6 (7.3, 42.5) | |||
| Hemozoin | |||||||||
| No | 52 | 24.0 (16.5, 34.8) | .009 | 43 | 60.0 (35.7, 100.9) | .006 | 16 | 8.6 (2.0, 37.8) | .317 |
| Yes | 45 | 10.6 (6.4, 17.5) | 39 | 17.1 (8.0, 36.3) | 33 | 20.9 (7.4, 59.3) | |||
| Dyserythropoiesis | |||||||||
| No | 30 | 18.9 (11.9, 29.9) | .479 | 25 | 27.5 (11.6, 65.5) | .753 | 7 | 13.4 (0.5, 376.1) | .885 |
| Yes | 70 | 14.8 (9.9, 22.1) | 61 | 32.5 (18.3, 57.7) | 45 | 11.3 (4.9, 25.9) | |||
| . | . | Relative transcript levels (×100)* . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Early . | . | Intermediate . | . | Mature . | |||
| . | n . | GM (95%CI) . | P . | n . | GM (95%CI) . | P . | n . | GM (95%CI) . | P . |
| Age (months) | |||||||||
| ≤20 | 76 | 17.2 (12.0, 24.9) | .296 | 61 | 40.8 (23.2, 72.0) | .314 | 35 | 8.5 (3.2, 22.7) | .200 |
| >20 | 53 | 12.8 (8.2, 19.8) | 48 | 26.3 (13.4, 51.4) | 35 | 20.3 (7.8, 52.6) | |||
| Sex | |||||||||
| Male | 73 | 14.9 (10.2, 21.9) | .871 | 61 | 29.9 (16.7, 53.3) | .541 | 39 | 13.6 (5.7, 32.4) | .90 |
| Female | 56 | 15.7 (10.3, 23.7) | 48 | 39.1 (20.2, 75.5) | 31 | 12.5 (4.1, 38.5) | |||
| Fever | |||||||||
| No | 52 | 21.0 (13.6, 32.4) | .061 | 42 | 48.0 (25.7, 89.4) | .195 | 26 | 40.6 (13.2, 125.1) | .009 |
| Yes | 77 | 12.3 (8.5, 17.7) | 67 | 26.9 (15.0, 48.3) | 44 | 6.7 (3.0, 15.1) | |||
| CRP† | |||||||||
| Low | 64 | 14.1 (9.2, 21.6) | .880 | 51 | 31.3 (15.9, 61.5) | .790 | 26 | 4.5 (1.5, 13.3) | .016 |
| High | 61 | 14.7 (10.2, 21.3) | 55 | 35.2 (19.6, 63.5) | 41 | 23.5 (10.1, 54.7) | |||
| Severe anemia | |||||||||
| No | 71 | 16.2 (11.2, 23.4) | .639 | 59 | 31.8 (16.8, 60.1) | .776 | 24 | 2.7 (0.9, 7.9) | <.001 |
| Yes | 58 | 14.2 (9.2, 21.9) | 50 | 36.0 (20.2, 64.2) | 46 | 30.1 (13.8, 65.4) | |||
| EPO‡ | |||||||||
| Low | 70 | 14.3 (9.9, 20.6) | .898 | 60 | 26.1 (14.2, 47.9) | .236 | 31 | 3.8 (1.4, 10.2) | .002 |
| High | 56 | 14.8 (9.6, 23.0) | 47 | 44.2 (23.5, 83.1) | 37 | 30.1 (12.8, 70.8) | |||
| Asexual parasitemia (BM)¶ | |||||||||
| Submic | 76 | 21.9 (15.9, 30.2) | .001 | 62 | 60.1 (37.9, 95.4) | <.001 | 29 | 9.7 (3.2, 29.3) | .386 |
| Patent | 44 | 8.4 (5.0, 14.1) | 40 | 10.7 (4.9, 23.3) | 35 | 17.6 (7.3, 42.5) | |||
| Hemozoin | |||||||||
| No | 52 | 24.0 (16.5, 34.8) | .009 | 43 | 60.0 (35.7, 100.9) | .006 | 16 | 8.6 (2.0, 37.8) | .317 |
| Yes | 45 | 10.6 (6.4, 17.5) | 39 | 17.1 (8.0, 36.3) | 33 | 20.9 (7.4, 59.3) | |||
| Dyserythropoiesis | |||||||||
| No | 30 | 18.9 (11.9, 29.9) | .479 | 25 | 27.5 (11.6, 65.5) | .753 | 7 | 13.4 (0.5, 376.1) | .885 |
| Yes | 70 | 14.8 (9.9, 22.1) | 61 | 32.5 (18.3, 57.7) | 45 | 11.3 (4.9, 25.9) | |||
P values are from Student t test using log transformed values.
CI, confidence interval; GM, geometric mean; submic, submicroscopic infections.
Ratio (×100) between copy numbers of gametocyte transcripts and P falciparum ubiquitin-conjugating enzyme transcript.
Median: 7.44 mg/dL.
Median: 88.65 U/L.
Submicroscopic if parasites were detected only by qPCR.
Demographic and clinical parameters associated with prevalence and relative transcript levels of early, intermediate, and mature gametocyte stages in bone marrow estimated by backward regression models
| . | n . | Significant variables . | Effect size (95% CI) . | P . |
|---|---|---|---|---|
| Without imputation | ||||
| Prevalences | ||||
| Early | 86 | — | ||
| Intermediate | 86 | — | ||
| Mature | 86 | Severe anemia | 2.94 (1.01, 8.61) | .049 |
| Dyserythropoiesis | 6.31 (1.75, 22.75) | .005 | ||
| Microscopic infection | 4.54 (1.45, 14.21) | .009 | ||
| Levels | ||||
| Early | 96 | Hemozoin | 0.43 (0.23, 0.78) | .007 |
| Intermediate | 81 | Microscopic infection | 0.21 (0.083, 0.51) | .001 |
| Mature | 67 | Severe anemia | 18.37 (5.53, 60.9) | <.001 |
| Fever | 0.26 (0.079, 0.83) | .026 | ||
| With imputation | ||||
| Prevalences | ||||
| Early | 136 | — | ||
| Intermediate | 136 | — | ||
| Mature | 136 | Severe anemia | 3.57 (1.49, 8.53) | .004 |
| Dyserythropoiesis | 6.21 (2.24, 17.25) | <.001 | ||
| Microscopic infection | 4.09 (1.58, 10.60) | .004 | ||
| Levels | ||||
| Early | 129 | Microscopic infection | 0.41 (0.23, 0.73) | .003 |
| Intermediate | 109 | Microscopic infection | 0.20 (0.083, 0.48) | <.001 |
| Mature | 70 | Severe anemia | 11.41 (3.37, 38.58) | <.001 |
| Fever | 0.16 (0.049, 0.54) | .003 | ||
| . | n . | Significant variables . | Effect size (95% CI) . | P . |
|---|---|---|---|---|
| Without imputation | ||||
| Prevalences | ||||
| Early | 86 | — | ||
| Intermediate | 86 | — | ||
| Mature | 86 | Severe anemia | 2.94 (1.01, 8.61) | .049 |
| Dyserythropoiesis | 6.31 (1.75, 22.75) | .005 | ||
| Microscopic infection | 4.54 (1.45, 14.21) | .009 | ||
| Levels | ||||
| Early | 96 | Hemozoin | 0.43 (0.23, 0.78) | .007 |
| Intermediate | 81 | Microscopic infection | 0.21 (0.083, 0.51) | .001 |
| Mature | 67 | Severe anemia | 18.37 (5.53, 60.9) | <.001 |
| Fever | 0.26 (0.079, 0.83) | .026 | ||
| With imputation | ||||
| Prevalences | ||||
| Early | 136 | — | ||
| Intermediate | 136 | — | ||
| Mature | 136 | Severe anemia | 3.57 (1.49, 8.53) | .004 |
| Dyserythropoiesis | 6.21 (2.24, 17.25) | <.001 | ||
| Microscopic infection | 4.09 (1.58, 10.60) | .004 | ||
| Levels | ||||
| Early | 129 | Microscopic infection | 0.41 (0.23, 0.73) | .003 |
| Intermediate | 109 | Microscopic infection | 0.20 (0.083, 0.48) | <.001 |
| Mature | 70 | Severe anemia | 11.41 (3.37, 38.58) | <.001 |
| Fever | 0.16 (0.049, 0.54) | .003 | ||
CI, confidence interval.
In contrast to mature stages, prevalence and levels of immature gametocytes were not found to be associated with severe anemia nor dyserythropoiesis (Tables 4-6). Relative transcript copy numbers of early and intermediate gametocytes were increased in children with submicroscopic infections in the bone marrow (Table 6) and decreased with increasing parasite densities (early: ρ = −0.344, P < .001; intermediate: ρ = −0.217, P = .011).
Discussion
This is the first ex vivo study on malaria-exposed children to apply molecular detection and stage differentiation of P falciparum gametocytes in the bone marrow, and to highlight the unexpectedly high prevalence of sexual stages as well as abundance of immature stages in this site compared with peripheral blood. The results also suggest a relationship between hematological disturbances and gametocyte development in the bone marrow.
In this study, practically every P falciparum-infected child carried gametocytes in the bone marrow (98%) and peripheral blood (100%). Most of these gametocyte-positive samples (78% in bone marrow and 88% in peripheral blood) were negative by microscopy, demonstrating the high prevalence of submicroscopic low-density gametocytemias in human hosts. Such underdetection in peripheral blood is concordant with a meta-analysis of 5 studies showing that the prevalence of gametocytes detected by microscopy in peripheral blood was only 2.8% to 26.6% of that detected by PCR.34 Early-stage gametocytes in bone marrow were particularly susceptible to underdetection by microscopy, probably because of the difficulty in distinguishing them from trophozoites35 and other nucleated host cells. Given that submicroscopic gametocyte infections are capable of infecting mosquitoes and contributing to transmission,36 these results suggest a higher potential for malaria transmission than previously observed in microscopically based studies.2
qPCR-based detection of sexual stage-specific transcripts showed that immature gametocytes were 5 times more prevalent and also more abundant in bone marrow compared with peripheral blood. This in contrast to the more mature gametocytemias observed in peripheral blood, although mature gametocytes were still observed in the blood circulating in the bone marrow. The very low transcript levels of immature gametocytes in the peripheral blood of 20% of the children suggest that the highly sensitive qPCR might be detecting residual early sexual stages in the bloodstream that may have escaped from the bone marrow. These results are in agreement with recent findings suggesting that mechanical properties of erythrocytes infected by immature gametocyte stages (ie, decreased deformability leading to the retention of immature gametocytes within the marrow)37,-39 may play a role in their localization in the bone marrow. However, it cannot be ruled out that cytoadhesion of erythrocytes infected by sexually committed trophozoites to specific receptors in the bone marrow may contribute to the enrichment of early sexual stages in this organ. Selective accumulation of immature gametocytes in bone marrow during the 8 to 12 days they need for maturation may provide them with a better niche for survival than the lumen of small vessels if, for example, their clearance is reduced in the special immune environment of the bone marrow.40 However, it is still not clear if gametocytogenesis can be induced in bone marrow by the high concentration of erythrocyte progenitors41,42 or other soluble factors43 or alternatively gametocytes are produced elsewhere and sequester in the bone marrow.
This study showed an elevated carriage of mature gametocytes in peripheral blood of P falciparum-infected children with severe anemia,2,44,45 representing an important infectious reservoir given the high numbers of anemic children in Mozambique (3.8 million children younger than age 10 years, 11.5% of them with severe anemia46 ) and in other African countries where malaria is endemic.47,48 Several interpretations for the observed association between gametocytemia and severe anemia are possible: (1) factors associated with severe anemia such as an increased erythropoiesis and/or the elevated production of EPO42,43,49,50 may stimulate the maturation of gametocytes in bone marrow and increase their release into peripheral blood; (2) long-term infections or high asexual parasite densities, as observed in this and other studies,51 leading to severe anemia may provide a longer time for gametocyte maturation; or (3) parasite sequestration in the bone marrow may alter or inhibit the differentiation of hematopoietic progenitors and increase the risk of severe anemia. Other factors such as high temperatures or increased levels of cytokines during febrile paroxysms may also contribute to eliminating mature gametocytes or delay the maturation of early stages, as suggested by the observation of reduced levels of mature stages among children with fever.44,45,52 In contrast to mature stages, prevalence and levels of early sexual stages were independent of severe anemia, dyserythropoiesis, or fever in the children in this study. Moreover, the univariate analysis found that relative levels of developing gametocytes decreased with increasing parasite densities in the bone marrow. These observations suggest that the relative contribution of immature gametocytes to the pool of parasites in peripheral blood is reduced in high-density infections, and that the genesis of gametocytes is not related to hematological disturbances.
This study has 2 main limitations. First, the analysis was restricted to anemic children. Dynamics of P falciparum sexual stages in children with other disease spectrum (ie, asymptomatic) as well as older children and adults with higher levels of antimalarial immunity stills needs to be addressed at the molecular level. Second, gametocyte stages in tissues other than bone marrow and peripheral blood were not assessed in this ex vivo study. Therefore it cannot be ruled out that other organs of the host may be implicated in the maturation of P falciparum sexual stages. However, a recent autopsy study provides independent evidence for the malaria sexual stage development in the hematopoietic system of the bone marrow (R.J., Sandra K. Nilsson, Jacqui Montgomery, Selasi Dankwa, Elizabeth Egan, Belinda Morahan, Karl B. Seydel, Lucia Bertuccini, Pietro Alano, Kim C. Williamson, Manoj T. Duraisingh, Terrie E. Taylor, Danny A. Milner, M.M., manuscript submitted September 2013), supporting the concept of bone marrow as a major source of immature gametocytes.
In summary, the qPCR-based detection of stage-specific gametocyte transcripts shows an enrichment of immature gametocytes in bone marrow compared with peripheral blood demonstrating their enrichment in this tissue. In addition, this study shows that almost all P falciparum-infected anemic children were gametocyte carriers potentially contributing to transmission, and points out a relationship between severe anemia, dyserythropoiesis, and gametocyte maturation in the bone marrow rather than with genesis of gametocytes. These findings indicate that strategies targeting gametocytes should take into account the high prevalence of low-density gametocytemias that are undetected by microscopy and the increased contribution of severely anemic children to the P falciparum-transmissible reservoir.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the children and their parents/guardians for their participation in the study, the staff of the Manhiça District Hospital and the Centro de Investigação em Saúde de Manhiça (CISM) for their work and dedication, and Alfred Cortés, Manuel Llinás, and Silvie Huijben for their useful comments and suggestions.
This study received financial support from the Agencia de Cooperación Internacional de Las Illes Balears, the Fundación Ramón Areces, and the Bill and Melinda Gates Foundation (Malaria Transmission Epidemiology Project). The CISM receives core support from the Spanish Agency for International Cooperation and Development. The Walter and Eliza Hall Institute receives Victorian State Government Operational Infrastructure Support and Australian Government National Health and Medical Research Council (NHMRC) Independent Research Institutes Infrastructure Support Scheme. L.S. received support from an NHMRC Program Grant; I.M. from an NHMRC Senior Research Fellowship; A.M.-T. from the Secretaría Nacional de Ciencia, Tecnología e Innovación de la República de Panamá; M.M. from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (R01A107755801); and A.M. from the Instituto de Salud Carlos III (CP-04/00220).
Authorship
Contribution: R.A., A.M.-T., A.H.A., R.J., I.M., M.M., C. Menéndez., L.S., and A.M. contributed to conception and design of the study; R.A., A.M.-T., A.H.A., R.J., C. Moraleda, E.M., P.L.A., C. Menéndez., P.C., and A.N. contributed to acquisition of data; R.A., I.M., C.S.N.L.W.S., and A.M. analyzed the data; all authors contributed to interpretation of data; R.A., A.M.-T., A.M., and C. Menéndez drafted the article; and all authors read, revised the article critically for important intellectual content, and gave final approval of the version to be published.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alfredo Mayor, Barcelona Centre for International Heath Research (CRESIB, Hospital Clínic - University of Barcelona), Rosselló 153, CEK 1st Floor, E-08036, Barcelona, Spain; e-mail: AGMAYOR@clinic.ub.es.