Key Points
Otlertuzumab (formerly TRU-016) has modest single-agent activity in symptomatic treated and untreated CLL.
Otlertuzumab demonstrates an acceptable safety profile, providing rationale for combination with other effective CLL therapies.
Abstract
Otlertuzumab is a novel humanized anti-CD37 protein therapeutic. This study evaluated the safety of otlertuzumab administered intravenously to patients with chronic lymphocytic leukemia (CLL). Otlertuzumab was administered weekly for up to 8 weeks followed by 1 dose per month for 4 months ranging from 0.03 to 20 mg/kg in the dose-escalation phase and 10 to 30 mg/kg in the dose-expansion phase. Responses were determined by using the 1996 National Cancer Institute (NCI-96) and 2008 International Workshop on Chronic Lymphocytic Leukaemia (IWCLL) criteria. Fifty-seven patients were treated in the dose-escalation phase and 26 in the dose-expansion phase. A maximum-tolerated dose was not identified. Response occurred in 19 (23%) of 83 treated patients by NCI-96 criteria. All responses were partial and occurred more commonly in patients with symptomatic untreated CLL (6/7) or 1 to 2 prior therapies (12/28) vs 3 or more therapies (1/48). Twenty percent (12/61) with serial computed tomography scan assessment had a response per IWCLL criteria. The most frequent adverse events were infusion reactions, fatigue, nausea, and diarrhea and were not dose related. Otlertuzumab was well tolerated, and modest clinical activity was observed. Otlertuzumab warrants further evaluation in combination with other agents for the treatment of CLL. This trial was registered at www.clinicaltrials.gov as #NCT00614042.
Introduction
Chronic lymphocytic leukemia (CLL) is a heterogeneous disease, primarily afflicting the elderly. Treatment of this disease has focused on chemoimmunotherapy, because the addition of rituximab to chemotherapy has improved response rates, response durations, and overall survival.1-3 Unfortunately, many elderly patients are not candidates for chemoimmunotherapy because of its toxicity and because single-agent fludarabine lacks benefit in individuals older than age 65 years.4,5 Additionally, patients inevitably relapse after fludarabine-based chemoimmunotherapy, making identification of new treatments with novel targets or methods of action necessary.3,4,6,7
CD37 is one such potential alternative target for antibody-directed therapy. CD37 is a member of the tetraspanin superfamily of molecules.8,9 Studies using CD37-deficient mice suggest that CD37 is involved in the regulation of B-cell function but is not required for B-cell development.9 CD37 is a heavily glycosylated cell surface protein expressed constitutively at high levels on human B cells and on transformed human B-cell leukemia and lymphoma cells.10-13 CD37 is not expressed on pro-B cells or terminally differentiated plasma cells; is either absent or expressed weakly on normal T cells, natural killer cells, monocytes, and neutrophils; and is absent from natural platelets and erythrocytes.14 CD37 is considered to be a lineage-specific marker of mature human B cells restricted to the surface of B lymphocytes and therefore represents a unique therapeutic target.
Until recently, only minimal effort has been directed toward CD37 immunotherapy. Kaminski et al15 and Press et al16 reported clinical activity of a radiolabeled antibody against CD37. Heider et al17 chimerized a high-affinity mouse antibody to CD37, and this Fc-engineered monoclonal antibody has been reported to produce B-cell–depleting activity in several in vitro systems and has pharmacodynamic and antitumor effects in animal models.
Otlertuzumab (formerly known as TRU-016) is a CD37-specific, single-chain, homodimeric therapeutic protein built on the ADAPTIR (modular protein technology) platform, consisting of antibody-derived, single-chain variable fragments linked to immunoglobulin (Ig) constant domains.18 ADAPTIR molecules such as otlertuzumab are similar to antibodies in functionality and pharmacokinetic (PK) properties but are smaller and, because of their different geometry, have the potential for differential signaling properties. A preclinical study with a murine anti-CD37 ADAPTIR molecule, SMIP-016, revealed superior in vitro natural killer cell–mediated antibody-dependent cellular cytotoxicity (ADCC) against primary human CLL cells compared with rituximab.18 In vivo studies with several lymphoma xenograft models supported the in vivo activity of SMIP-016 as monotherapy and in combination with therapies such as bendamustine and rituximab.19,20 Furthermore, SMIP-016 induces apoptosis of CLL cells directly in a tyrosine phosphorylation–dependent manner that suggests an alternative signaling mechanism of action compared with rituximab in which inhibition of tyrosine phosphorylation enhances cytotoxicity.21 A recent publication demonstrated that CD37 has both ITIM and ITAM-like signaling activity, and ligation of this antigen by SMIP-016 prompts recruitment of the phosphatase SHP1, inhibition of the PI3-kinase pathway, and upregulation of BIM, which is responsible for apoptosis mediated by this agent.22 A recent study has demonstrated that CD20 antibodies mediate death independent of SHP1 by inhibiting B-cell receptor signaling.23 Given that the mechanism of killing through CD37 is distinct from that of CD20 and the selective binding of otlertuzumab to B cells and promising in vivo activity of SMIP-016, the fully humanized otlertuzumab built on the ADAPTIR platform was moved into the clinic for initial phase 1 testing described herein.
Patients and methods
Patients
In the dose-escalation stage of this study, eligible patients were those with symptomatic CLL/small lymphocytic lymphoma who had relapsed or refractory disease following 1 or more previous treatment regimens. In the expansion phase of the study, patients with treated or untreated symptomatic CLL were eligible. In both the phase 1 dose-escalation and dose-expansion phases, the requirements were active disease requiring therapy as put forth by the National Cancer Institute 1996 (NCI-96) guidelines24 ; age ≥18 years; Eastern Cooperative Oncology Group performance status of ≤2; life expectancy >3 months; serum creatinine, total bilirubin, serum glutamic-oxaloacetic transaminase, and serum glutamic-pyruvic transaminase ≤2.0 × upper limit of normal; absolute neutrophil count (ANC) ≥500/mm3 (patients with lymphocyte count >100 × 109/L were allowed irrespective of ANC); platelets ≥30 000/mm3; and no anticancer therapy or surgery within 30 days of treatment. Patients were not eligible if they had received alemtuzumab or radioimmunotherapy within 12 weeks of enrollment, had an additional malignancy that could confound interpretation of study result, or had known positive serology for human immunodeficiency virus, hepatitis C, or hepatitis B surface antigen or hepatitis B core antibody. The study protocol was approved by an independent ethics committee or institutional review board at each study site. All patients provided written informed consent to participate in the study according to the Declaration of Helsinki.
Study design and treatment
The study was divided into 2 stages: dose-escalation and dose-expansion. Otlertuzumab was administered by intravenous infusion at the doses and schedule shown in Table 1. Dose escalation used an accelerated titration design for dose groups up to the 1-mg/kg dose (cohort 4), with 1 patient treated per cohort, provided that no patient experienced a grade 2 or greater toxicity attributed to otlertuzumab. For cohort 4 and above, a standard 3 × 3 design was used. Dose-limiting toxicity (DLT) included grade 3 thrombocytopenia or anemia or grade 4 neutropenia (as described by NCI-96 criteria) that did not resolve within 7 days; grade 3 nonhematologic drug-related adverse event (NCI Common Terminology Criteria for Adverse Events [CTCAE], Version 3); or any adverse event without clear evidence to support an alternative causality other than otlertuzumab requiring more than a 14-day dose delay for recovery back to baseline. During dose escalation, cohorts were included that used dosing 3 times in the first week as a means to “load” otlertuzumab.
Dose and schedule
| Cohort . | No. . | Dose (mg/kg) . | Schedule . |
|---|---|---|---|
| 1 | 1 | 0.03 | Weekly ×4 |
| 2 | 1 | 0.1 | Weekly ×4 |
| 3 | 1 | 0.3 | Weekly ×4 |
| 4 | 3 | 1.0 | Weekly ×4 |
| 5 | 4 | 3.0 | Weekly ×4 |
| 6 | 7 | 6.0 | Weekly ×4 |
| 7 | 8 | 3.0 | Days 1, 3, and 5, then weekly ×3 |
| 8 | 8 | 10 | Cycle 1: 3 mg/kg day 1, 7 mg/kg day 3, then weekly ×3 |
| 9 | 8 | 15 | Cycle 1: 3 mg/kg day 1, 12 mg/kg day 3, then weekly ×3 |
| 10 | 9 | 20 | Cycle 1: 3 mg/kg day 1, 17 mg/kg day 3, then weekly ×3 |
| 11 | 4 | 6.0 | Days 1, 3, and 5, then once per week ×3 |
| 12 | 3 | 10 | Days 1, 3, and 5, then once per week ×3 |
| Expansion | 26 | 10, 20, or 30 | Once per week ×8, then once per month ×4 |
| Cohort . | No. . | Dose (mg/kg) . | Schedule . |
|---|---|---|---|
| 1 | 1 | 0.03 | Weekly ×4 |
| 2 | 1 | 0.1 | Weekly ×4 |
| 3 | 1 | 0.3 | Weekly ×4 |
| 4 | 3 | 1.0 | Weekly ×4 |
| 5 | 4 | 3.0 | Weekly ×4 |
| 6 | 7 | 6.0 | Weekly ×4 |
| 7 | 8 | 3.0 | Days 1, 3, and 5, then weekly ×3 |
| 8 | 8 | 10 | Cycle 1: 3 mg/kg day 1, 7 mg/kg day 3, then weekly ×3 |
| 9 | 8 | 15 | Cycle 1: 3 mg/kg day 1, 12 mg/kg day 3, then weekly ×3 |
| 10 | 9 | 20 | Cycle 1: 3 mg/kg day 1, 17 mg/kg day 3, then weekly ×3 |
| 11 | 4 | 6.0 | Days 1, 3, and 5, then once per week ×3 |
| 12 | 3 | 10 | Days 1, 3, and 5, then once per week ×3 |
| Expansion | 26 | 10, 20, or 30 | Once per week ×8, then once per month ×4 |
Weekly means once per week. Patients in cohorts 5 through 12 could receive 2 additional 4-week cycles if there was evidence of clinical benefit.
Patients who experienced a DLT during the first cycle (28 days) of therapy without recovery within protocol limits were removed from the trial. If the DLT resolved, the patient continued in the study at the previous lower dose level. Supportive allopurinol, valacyclovir or equivalent for antiviral prophylaxis, and infusion premedications (acetaminophen 650 mg orally and diphenhydramine 50 mg intravenously) were given prior to the infusion of otlertuzumab. Hydrocortisone (100 mg intravenously) was given prior to otlertuzumab during the first cycle of therapy.
Patients underwent clinical restaging on days 29, 57, 85, and 113 and were then assessed every 3 months until progression of disease, death, withdrawal from study, or completion of 2 years of follow-up evaluations. Computed tomography (CT) or magnetic resonance imaging (MRI) scans were required at baseline and at the end of treatment in both stages of the study. In the dose-expansion cohort, CT/MRI scans were repeated 2 months after treatment was completed to confirm the response. If the response (partial response [PR] or complete response) by complete blood count and clinical evaluation was confirmed by CT/MRI scans, then a bone marrow biopsy and aspirate were performed.
PK analyses
Serum samples for PK analyses were analyzed by a validated and sensitive enzyme-linked immunosorbent assay. Actual times after dose administration for individual patients were used in all PK calculations; however, nominal times were used for graphing PK data. Patients not receiving a full dose of otlertuzumab were excluded from PK parameter calculations such as mean maximum observed concentration (Cmax) and total area under the curve (AUC). Values for Cmax and time to reach Cmax (tmax) were obtained by direct inspection of data. Area under the concentration-time curve (AUCt) was determined by the log-linear trapezoidal rule from time 0 to the last observed concentration (Ct) at time t using GraphPad Prism Version 5.0 (GraphPad Software, San Diego, CA). Otlertuzumab PK parameters were estimated by using validated WinNonlin Professional Version 5.3 software (Pharsight Corporation, Mountain View, CA) with noncompartmental methods when a patient had sufficient late time points available for PK analysis. Individual concentration-time profiles were plotted, and the terminal disposition rate constant (λz) was determined by the log-linear regression of at least 3 points judged to be in the terminal phase. The apparent terminal elimination half-life (t1/2) was estimated by dividing the natural log of 2 by λz (0.693/λz). WinNonlin software was also used to estimate clearance (CL) and volume of distribution (Vz) parameters. Descriptive statistics, such as means, standard deviations, and precision (percent coefficient of variation [% CV]) were calculated for all variables by dose cohorts using Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA).
Toxicity and response criteria
Toxicity was assessed at each evaluation by using the NCI CTCAE Version 3 criteria. Response to therapy was assessed by using the NCI-96 criteria.24 In addition, response rates confirmed by CT scan, per the recommendations for clinical trials as part of the International Workshop for Chronic Lymphocytic Leukemia (IWCLL) 2008 criteria,25 are presented.
Statistical methods
Data analyses were based on descriptive statistics for the dose groups. For continuous variables, these statistics included the mean, median, standard deviation, minimum, and maximum. Final study analyses were conducted after the last patient completed study treatment. Response was assessed by using intent-to-treat analysis with the NCI-96 and IWCLL 2008 criteria. Progression-free survival was defined from the day of first treatment until time to documented disease progression using NCI-96 criteria. Duration of response was calculated for those patients achieving a complete response or PR. Duration was derived as the difference (in days) between the first date that response criteria were satisfied and the earliest date that objective criteria for disease progression or death were met. Event times were censored on the last date that valid tumor assessments were made for cases when a patient did not experience disease progression or death. The duration of best overall response was estimated by using the Kaplan-Meier method.
Results
Patients
Fifty-seven patients with CLL/small lymphocytic lymphoma were enrolled onto 12 dose-escalation cohorts and 26 patients were enrolled onto 4 dose-expansion cohorts (Table 1) at 8 sites between March 2008 and February 2011. Three patients in the dose-escalation cohorts treated at low doses were retreated in the dose-expansion phase of the trial. Demographic characteristics are summarized in Table 2 and include a median age of 66 years (range, 39 to 83 years); 33 patients (39.8%) were age 70 years or older. Fifty patients (60.2%) were Rai stage III to IV, and 52 (62.7%) had a β2-microglobulin >3 mg/L. The median number of prior regimens was 4 (range, 1 to 13) among relapsed patients. Twenty-five patients had del(17p13.1), 17 had del(11q22.3), and 4 had both deletions.
Demographics and baseline characteristics
| Characteristic . | Dose escalation (n = 57) . | Dose expansion (n = 26) . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| Sex | ||||
| Female | 20 | 35.1 | 12 | 46.2 |
| Male | 37 | 64.9 | 14 | 53.8 |
| Age | ||||
| Median | 66 | 69 | ||
| Range | 44-83 | 39-81 | ||
| ≥70 y | 21 | 36.8 | 12 | 46.2 |
| β2-microgobulin, mg/L | ||||
| 0-3 | 12 | 21.1 | 16 | 61.5 |
| 3.1-5 | 21 | 36.8 | 6 | 23.1 |
| 5.1-10 | 19 | 33.3 | 2 | 7.7 |
| >10 | 4 | 7.0 | 0 | |
| Unknown | 1 | 1.8 | 2 | 7.7 |
| ANC <1.5 × 109/L | 15 | 26.3 | 10 | 38.5 |
| Hemoglobin ≤11 g/dL | 32 | 56.1 | 13 | 50.0 |
| Platelets <100 000 × 109/L | 26 | 45.6 | 6 | 23.1 |
| Rai stage | ||||
| 0 | 1 | 1.8 | 3 | 11.5 |
| 1-2 | 17 | 29.8 | 11 | 42.3 |
| 3-4 | 39 | 68.4 | 11 | 42.3 |
| Not recorded | — | 1 | 3.8 | |
| Duration since diagnosis, y | ||||
| Median | 7 | 7 | ||
| Range | 1-21 | 0-33 | ||
| Time since last treatment, mo | ||||
| N/A | 7 | 26.9 | ||
| <6 | 25 | 43.9 | 7 | 26.9 |
| 6-12 | 14 | 24.6 | 1 | 3.8 |
| >12 | 18 | 31.6 | 11 | 42.3 |
| Any one lymph node >5 cm | 27 | 47.4 | 6 | 23.2 |
| Hepatomegaly | 2 | 3.5 | 1 | 3.8 |
| Splenomegaly | 13 | 22.8 | 7 | 26.9 |
| B symptoms | 25 | 78* | 17 | 65.4 |
| SLL | 6 | 10.5 | 0 | |
| ECOG PS | ||||
| 0 | 18 | 31.6 | 13 | 50.0 |
| 1 | 36 | 63.2 | 13 | 50.0 |
| 2 | 3 | 5.3 | 0 | |
| No. of previous therapies | ||||
| Median | 4 | 1 | ||
| Range | 1-13 | 0-11 | ||
| 0 | 0 | 7 | 26.9 | |
| 1-2 | 17 | 29.8 | 11 | 42.3 |
| 3-6 | 23 | 40.3 | 5 | 19.2 |
| 7-10 | 12 | 21.1 | 2 | 0.8 |
| >10 | 5 | 8.8 | 1 | 3.8 |
| Previous anti-CD20 therapy | ||||
| Median | 2 | 1 | ||
| Range | 0-7 | 0-9 | ||
| 0 | 5 | 8.8 | 12 | 46.2 |
| 1-2 | 31 | 54.3 | 8 | 30.8 |
| 3 | 8 | 14.0 | 3 | 11.5 |
| 4 | 5 | 8.8 | 0 | |
| ≥5 | 8 | 14.0 | 3 | 11.5 |
| FISH | ||||
| 17p | 23 | 2 | ||
| 13q | 29 | 10 | ||
| 11q | 14 | 3 | ||
| Characteristic . | Dose escalation (n = 57) . | Dose expansion (n = 26) . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| Sex | ||||
| Female | 20 | 35.1 | 12 | 46.2 |
| Male | 37 | 64.9 | 14 | 53.8 |
| Age | ||||
| Median | 66 | 69 | ||
| Range | 44-83 | 39-81 | ||
| ≥70 y | 21 | 36.8 | 12 | 46.2 |
| β2-microgobulin, mg/L | ||||
| 0-3 | 12 | 21.1 | 16 | 61.5 |
| 3.1-5 | 21 | 36.8 | 6 | 23.1 |
| 5.1-10 | 19 | 33.3 | 2 | 7.7 |
| >10 | 4 | 7.0 | 0 | |
| Unknown | 1 | 1.8 | 2 | 7.7 |
| ANC <1.5 × 109/L | 15 | 26.3 | 10 | 38.5 |
| Hemoglobin ≤11 g/dL | 32 | 56.1 | 13 | 50.0 |
| Platelets <100 000 × 109/L | 26 | 45.6 | 6 | 23.1 |
| Rai stage | ||||
| 0 | 1 | 1.8 | 3 | 11.5 |
| 1-2 | 17 | 29.8 | 11 | 42.3 |
| 3-4 | 39 | 68.4 | 11 | 42.3 |
| Not recorded | — | 1 | 3.8 | |
| Duration since diagnosis, y | ||||
| Median | 7 | 7 | ||
| Range | 1-21 | 0-33 | ||
| Time since last treatment, mo | ||||
| N/A | 7 | 26.9 | ||
| <6 | 25 | 43.9 | 7 | 26.9 |
| 6-12 | 14 | 24.6 | 1 | 3.8 |
| >12 | 18 | 31.6 | 11 | 42.3 |
| Any one lymph node >5 cm | 27 | 47.4 | 6 | 23.2 |
| Hepatomegaly | 2 | 3.5 | 1 | 3.8 |
| Splenomegaly | 13 | 22.8 | 7 | 26.9 |
| B symptoms | 25 | 78* | 17 | 65.4 |
| SLL | 6 | 10.5 | 0 | |
| ECOG PS | ||||
| 0 | 18 | 31.6 | 13 | 50.0 |
| 1 | 36 | 63.2 | 13 | 50.0 |
| 2 | 3 | 5.3 | 0 | |
| No. of previous therapies | ||||
| Median | 4 | 1 | ||
| Range | 1-13 | 0-11 | ||
| 0 | 0 | 7 | 26.9 | |
| 1-2 | 17 | 29.8 | 11 | 42.3 |
| 3-6 | 23 | 40.3 | 5 | 19.2 |
| 7-10 | 12 | 21.1 | 2 | 0.8 |
| >10 | 5 | 8.8 | 1 | 3.8 |
| Previous anti-CD20 therapy | ||||
| Median | 2 | 1 | ||
| Range | 0-7 | 0-9 | ||
| 0 | 5 | 8.8 | 12 | 46.2 |
| 1-2 | 31 | 54.3 | 8 | 30.8 |
| 3 | 8 | 14.0 | 3 | 11.5 |
| 4 | 5 | 8.8 | 0 | |
| ≥5 | 8 | 14.0 | 3 | 11.5 |
| FISH | ||||
| 17p | 23 | 2 | ||
| 13q | 29 | 10 | ||
| 11q | 14 | 3 | ||
ECOG PS, Eastern Cooperative Oncology Group performance score; FISH, fluorescence in situ hybridization; N/A, not applicable; SLL, small lymphocytic leukemia.
B symptoms were not collected in cohorts 1 through 7; symptoms were collected for 32 patients.
Treatment
The protocol, which originally allowed dosing for 1 month, was amended to allow dosing for up to 3 months in the dose-escalation cohort and dosing for up to 6 months in the dose-expansion cohort. Dose escalation was successful through all cohorts up to 30 mg/kg per week without identification of a maximum-tolerated dose (MTD). A total of 747 doses were administered to study patients; the median number of doses per individual was 9 (range, 1 to 18).
Toxicity observed
In the dose-escalation cohorts, 4 patients experienced 5 DLTs. No MTD was identified. The DLTs occurred across several dose cohorts, were not dose related, and did not meet the criteria for determining the MTD. These DLTs included grade 4 neutropenia at 6 mg/kg, grade 4 idiopathic thrombocytopenia at 3 mg/kg 3 times per week, and grade 4 neutropenia (2 patients) and grade 3 anemia at 15 mg/kg. Adverse events are summarized in Table 3. The most frequently reported adverse events (>20%) were fatigue, nausea, diarrhea, neutropenia, cough, chills, and pyrexia. Neutropenia, fatigue, thrombocytopenia, anemia, febrile neutropenia, and hypophosphatemia were the most frequent (>5%) grade 3 and 4 adverse events. Ten patients (12%) had adverse events that resulted in discontinuation of study drug, including neutropenia in 3 patients, infusion-related reaction in 2 patients, and idiopathic thrombocytopenic purpura, fatigue, septic shock, pneumonia, sepsis, pyrexia, and atrial fibrillation in 1 patient each. Severe infusion reactions occurred in 2 patients and were readily managed with treatment interruption. Adverse events interrupted infusion in 28.9% of patients. These events were chills in 9 patients, nausea in 5 patients, infusion reaction in 4 patients, vomiting and hypotension in 3 patients each, hot flashes and musculoskeletal pain in 2 patients each, and abdominal pain, anxiety, and injection site swelling in 1 patient each. Serious adverse events considered possibly related to study treatment were febrile neutropenia in 3 patients, atrial fibrillation in 2 patients, and neutropenia, herpes zoster, thrombocytopenia, pyrexia, appendicitis, pneumonia, diarrhea, staphylococcal infection, and catheter-related venous thrombosis in 1 patient each. Serious adverse clinically asymptomatic, transient mild-to-moderate hypophosphatemia of unclear etiology was noted on laboratory testing that was not related to dose or cycle. Levels of IgG, IgA, and IgM were not significantly changed from pre- to posttherapy. Additionally, there was no change in CD3+ T cells from pre- to posttherapy.
Adverse events occurring in >5% of patients (n = 83)
| Event . | All events . | Grade 3 to 4 events . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| Any event | 82 | 98.8 | 52 | 62.7 |
| Fatigue | 28 | 33.7 | 8 | 9.6 |
| Nausea | 26 | 31.3 | 1 | 1.2 |
| Diarrhea | 24 | 28.9 | 2 | 2.4 |
| Neutropenia | 20 | 24.1 | 14 | 19.3 |
| Cough | 19 | 22.9 | 1 | 1.2 |
| Chills | 17 | 20.5 | 1 | 1.2 |
| Pyrexia | 17 | 20.5 | 1 | 1.2 |
| Peripheral edema | 16 | 19.3 | 0 | 0 |
| Anemia | 13 | 15.7 | 4 | 4.8 |
| Back pain | 13 | 15.7 | 2 | 2.4 |
| Dyspnea | 13 | 15.7 | 3 | 3.6 |
| Thrombocytopenia | 12 | 14.5 | 6 | 7.2 |
| Vomiting | 10 | 12.0 | 0 | 0 |
| Muscle spasms | 10 | 12.0 | 0 | 0 |
| Headache | 10 | 12.0 | 0 | 0 |
| Night sweats | 10 | 12.0 | 0 | 0 |
| Abdominal pain | 9 | 10.8 | 1 | 1.2 |
| Constipation | 9 | 10.8 | 0 | 0 |
| Hypophosphatemia | 9 | 10.8 | 5 | 6.0 |
| Infusion-related reaction | 7 | 8.4 | 1 | 1.2 |
| Decreased appetite | 7 | 8.4 | 0 | 0 |
| Hyperglycemia | 7 | 8.4 | 2 | 2.4 |
| Upper respiratory tract infection | 6 | 7.2 | 0 | 0 |
| Hypocalcemia | 6 | 7.2 | 0 | 0 |
| Dizziness | 6 | 7.2 | 0 | 0 |
| Sinus congestion | 6 | 7.2 | 1 | 1.2 |
| Febrile neutropenia | 5 | 6.0 | 5 | 6.0 |
| Abdominal distension | 5 | 6.0 | 0 | 0 |
| Sinusitis | 5 | 6.0 | 0 | 0 |
| Hypokalemia | 5 | 6.0 | 2 | 2.4 |
| Arthralgia | 5 | 6.0 | 0 | 0 |
| Insomnia | 5 | 6.0 | 0 | 0 |
| Pollakiuria | 5 | 6.0 | 0 | 0 |
| Pharyngolaryngeal pain | 5 | 6.0 | 0 | 0 |
| Event . | All events . | Grade 3 to 4 events . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| Any event | 82 | 98.8 | 52 | 62.7 |
| Fatigue | 28 | 33.7 | 8 | 9.6 |
| Nausea | 26 | 31.3 | 1 | 1.2 |
| Diarrhea | 24 | 28.9 | 2 | 2.4 |
| Neutropenia | 20 | 24.1 | 14 | 19.3 |
| Cough | 19 | 22.9 | 1 | 1.2 |
| Chills | 17 | 20.5 | 1 | 1.2 |
| Pyrexia | 17 | 20.5 | 1 | 1.2 |
| Peripheral edema | 16 | 19.3 | 0 | 0 |
| Anemia | 13 | 15.7 | 4 | 4.8 |
| Back pain | 13 | 15.7 | 2 | 2.4 |
| Dyspnea | 13 | 15.7 | 3 | 3.6 |
| Thrombocytopenia | 12 | 14.5 | 6 | 7.2 |
| Vomiting | 10 | 12.0 | 0 | 0 |
| Muscle spasms | 10 | 12.0 | 0 | 0 |
| Headache | 10 | 12.0 | 0 | 0 |
| Night sweats | 10 | 12.0 | 0 | 0 |
| Abdominal pain | 9 | 10.8 | 1 | 1.2 |
| Constipation | 9 | 10.8 | 0 | 0 |
| Hypophosphatemia | 9 | 10.8 | 5 | 6.0 |
| Infusion-related reaction | 7 | 8.4 | 1 | 1.2 |
| Decreased appetite | 7 | 8.4 | 0 | 0 |
| Hyperglycemia | 7 | 8.4 | 2 | 2.4 |
| Upper respiratory tract infection | 6 | 7.2 | 0 | 0 |
| Hypocalcemia | 6 | 7.2 | 0 | 0 |
| Dizziness | 6 | 7.2 | 0 | 0 |
| Sinus congestion | 6 | 7.2 | 1 | 1.2 |
| Febrile neutropenia | 5 | 6.0 | 5 | 6.0 |
| Abdominal distension | 5 | 6.0 | 0 | 0 |
| Sinusitis | 5 | 6.0 | 0 | 0 |
| Hypokalemia | 5 | 6.0 | 2 | 2.4 |
| Arthralgia | 5 | 6.0 | 0 | 0 |
| Insomnia | 5 | 6.0 | 0 | 0 |
| Pollakiuria | 5 | 6.0 | 0 | 0 |
| Pharyngolaryngeal pain | 5 | 6.0 | 0 | 0 |
Response to therapy
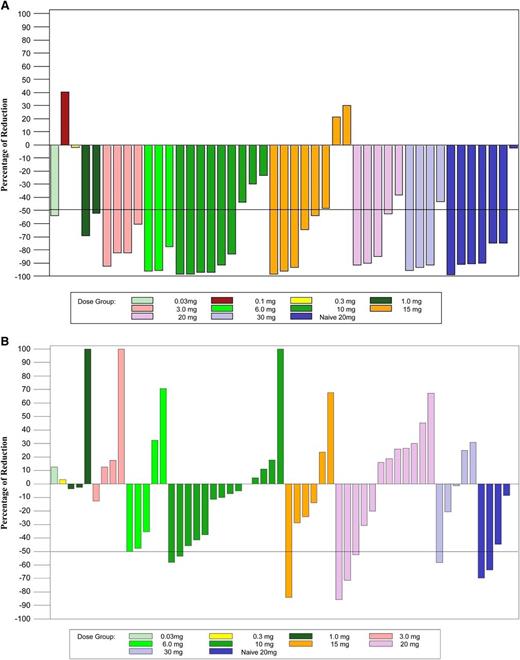
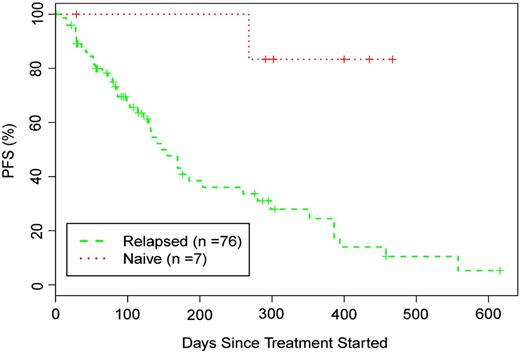
Otlertuzumab reduced peripheral lymphocytes in a dose-dependent relationship. Lymphocyte reduction ≥50% was observed in 75.5% of patients (34/45) with a baseline lymphocyte count >5000/μL. Figure 1A is a plot of reduction of lymphocyte counts in patients who received otlertuzumab once per week for 4 weeks. By using the predetermined NCI-96 criteria,24 the overall response for all 83 enrolled patients was 23% (19/83). The response in patients with del17p was 16% (4/25); with del11q, 6% (1/17); and with both deletions, 25% (1/4). Treatment response varied by prior treatment status with 6 (86%) of 7 patients in the symptomatic previously untreated group responding, whereas for patients with prior therapy, only 13 (17%) of 76 responded. These responses occurred most commonly in those receiving 1 to 2 prior therapies (12/28; 43%) compared with 3 or more therapies (1/48; 2%). Including 15 patients who had not progressed at their last assessment, response duration ranged from 184 to 372 days with a median of 268 days in previously untreated patients and from 68 to 431 days with a median of 197 days in patients with relapsed disease (Figure 2). Progression-free survival for all patients was a median of 97 days; 289 days for those with a PR; 91 days for those with stable disease; and 28 days for those with progressive disease.
Lymphocytes and lymph node size from baseline to end of treatment. (A) The total absolute lymphocyte count was compared from day 1 prior to the first dose of otlertuzumab to the end of treatment visit which occurred 1 to 2 weeks after the last dose of otlertuzumab. A decrease of greater than 50% was observed at most dose levels and was present overall in 75.5% of patients with an elevated lymphocyte count at day 1. Patients who received otlertuzumab weekly for 4 weeks are included in this graph. (B) Lymph node sum of product diameters from CT scans obtained during screening were compared with CT scans at the end of treatment. A reduction of 50% or greater was observed at 6 mg/kg and higher. Patients who received otlertuzumab weekly for 4 weeks are included in this graph.
Lymphocytes and lymph node size from baseline to end of treatment. (A) The total absolute lymphocyte count was compared from day 1 prior to the first dose of otlertuzumab to the end of treatment visit which occurred 1 to 2 weeks after the last dose of otlertuzumab. A decrease of greater than 50% was observed at most dose levels and was present overall in 75.5% of patients with an elevated lymphocyte count at day 1. Patients who received otlertuzumab weekly for 4 weeks are included in this graph. (B) Lymph node sum of product diameters from CT scans obtained during screening were compared with CT scans at the end of treatment. A reduction of 50% or greater was observed at 6 mg/kg and higher. Patients who received otlertuzumab weekly for 4 weeks are included in this graph.
Progression-free survival of patients treated with otlertuzumab based on treatment status.
Progression-free survival of patients treated with otlertuzumab based on treatment status.
Response rate was lower when including only responses confirmed by CT scan measurements per the IWCLL response criteria for clinical trials. Screening and posttreatment CT scans were available for 40 patients in the dose-escalation cohort and for 21 patients in the dose-expansion cohort. Figure 1B is a plot of lymph node sum of product diameters in patients who received otlertuzumab once per week for 4 weeks. The reduction in lymph node sum of product diameters was first evident in the 1-mg/kg dose cohort and appears to be dose dependent. Twelve of these 61 patients had a reduction in lymph nodes of ≥50% by CT scan. By using the IWCLL response criteria, the overall response rate was 19.7% (all PRs). Improvement in cytopenias (>50%) was observed, along with hemoglobin 14% (6/44), platelets 39% (12/31), and ANC 33% (8/24). Minimal residual disease was not assessed in this study.
Development of antidrug antibodies
All patients with late postdose serum samples available for testing were screened for development of antidrug antibodies (ADAs), and 4 patients (5%) developed detectable levels of ADAs. Only one individual had a significant titer (1:14 580); this patient received 4 weekly 15 mg/kg doses and discontinued for progressive disease without a response in lymphocyte count. The patient had no adverse events that would suggest an allergic reaction. The patient’s PK parameters were altered, with a t1/2 approximately 60% less than the mean of the other patients.
PK
Table 4 shows mean PK parameters for cycle 1 by cohort. Serum concentrations of otlertuzumab could be detected in patient samples at all dose levels for time points collected during and 30 minutes after otlertuzumab infusion, which corresponded to the Cmax value. Otlertuzumab could be detected in sera samples for Cmin evaluation at doses of 0.3 mg/kg and higher. Moderate variability was observed in some dose cohorts, but this was likely due to the large variability in tumor burden which would act as an antigen sink for circulating otlertuzumab. A dose-proportional response was observed for doses of 10 to 30 mg/kg. Concentration vs time curves were similar across patient populations at the same doses.
Summary of PK parameters for otlertuzumab (cycle 1)
| Dose (mg/kg) . | No. of patients . | Cmax (μg/mL) . | Half-life (h) . | CL (mL/h/kg) . | AUC all (h⋅mg/mL) . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean . | %CV . | Mean . | %CV . | Mean . | %CV . | Mean . | %CV . | ||
| Dose-escalation cohorts | |||||||||
| 1 | 2 | 19.5 | 24.2 | 133.7 | 4.9 | 1.62 | 9.7 | 4 846 | 39.7 |
| 3 | 2 | 41.5 | 26.7 | 208.1 | 68.6 | 2.87 | 33.6 | 18 333 | 80.4 |
| 6 | 1 | 128.6 | 25.7 | 271.9 | 0 | 2.1 | 0 | 42 683 | 45.2 |
| 3* | 4 | 138.0 | 64.2 | 178.6 | 65.7 | 0.306 | 44.5 | 45 728 | 69.5 |
| 10 | 7 | 435.7 | 23.3 | 221.7 | 38.5 | 0.473 | 129.5 | 413 319 | 53.0 |
| 15 | 6 | 703.7 | 51.4 | 176.3 | 25.6 | 0.262 | 62.8 | 593 848 | 132.6 |
| 20 | 9 | 1 081.1 | 49.9 | 177.9 | 30.5 | 0.129 | 623 | 831 562 | 66.0 |
| 6* | 3 | 367.5 | 31.6 | 247.8 | 38.0 | 0.129 | 58.9 | 37 625 | 58.5 |
| 10* | 1 | 202 | 28.4 | 121.2 | 0 | 0.7 | 0 | 42 961 | 71.1 |
| Dose-expansion cohorts | |||||||||
| 10 | 3 | 461.5 | 30.2 | 236.7 | 24.6 | 1.32 | 157.7 | 602 581 | 69.4 |
| 20 | 2 | 1 175.6 | 18.4 | 129.7 | 0.5 | 0.05 | 33.5 | 857 111 | 50.0 |
| 30 | 6 | 1 756.5 | 36.2 | 217.0 | 39.6 | 0.53 | 113.8 | 2 025 904 | 41.9 |
| Dose (mg/kg) . | No. of patients . | Cmax (μg/mL) . | Half-life (h) . | CL (mL/h/kg) . | AUC all (h⋅mg/mL) . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean . | %CV . | Mean . | %CV . | Mean . | %CV . | Mean . | %CV . | ||
| Dose-escalation cohorts | |||||||||
| 1 | 2 | 19.5 | 24.2 | 133.7 | 4.9 | 1.62 | 9.7 | 4 846 | 39.7 |
| 3 | 2 | 41.5 | 26.7 | 208.1 | 68.6 | 2.87 | 33.6 | 18 333 | 80.4 |
| 6 | 1 | 128.6 | 25.7 | 271.9 | 0 | 2.1 | 0 | 42 683 | 45.2 |
| 3* | 4 | 138.0 | 64.2 | 178.6 | 65.7 | 0.306 | 44.5 | 45 728 | 69.5 |
| 10 | 7 | 435.7 | 23.3 | 221.7 | 38.5 | 0.473 | 129.5 | 413 319 | 53.0 |
| 15 | 6 | 703.7 | 51.4 | 176.3 | 25.6 | 0.262 | 62.8 | 593 848 | 132.6 |
| 20 | 9 | 1 081.1 | 49.9 | 177.9 | 30.5 | 0.129 | 623 | 831 562 | 66.0 |
| 6* | 3 | 367.5 | 31.6 | 247.8 | 38.0 | 0.129 | 58.9 | 37 625 | 58.5 |
| 10* | 1 | 202 | 28.4 | 121.2 | 0 | 0.7 | 0 | 42 961 | 71.1 |
| Dose-expansion cohorts | |||||||||
| 10 | 3 | 461.5 | 30.2 | 236.7 | 24.6 | 1.32 | 157.7 | 602 581 | 69.4 |
| 20 | 2 | 1 175.6 | 18.4 | 129.7 | 0.5 | 0.05 | 33.5 | 857 111 | 50.0 |
| 30 | 6 | 1 756.5 | 36.2 | 217.0 | 39.6 | 0.53 | 113.8 | 2 025 904 | 41.9 |
AUC all, area under the concentration-time curve from time 0 to the last measurable time point; CL, serum clearance; Cmax, maximum observed concentration; CV, coefficient of variation. Cycle 1 AUC, area under the concentration-time curve from time 0 to approximately day 29.
Dosed days 1, 3, and 5, then weekly ×3.
The PK of otlertuzumab appears to be dose proportionate in terms of Cmax and Cmin, since increasing doses from 0.3 to 30 mg/kg results in equivalent increases in serum concentration. However, increasing doses of otlertuzumab did not seem to influence half-life. The mean terminal elimination half-life for otlertuzumab was 8 days for all dose-escalation cohorts compared with 9 days for the dose-expansion cohorts. There appeared to be no PK differences between responders and nonresponders.
Discussion
Herein, we report the results of a large, multicenter, phase 1, first-in-human study of the anti-CD37 ADAPTIR molecule, otlertuzumab, in CLL. This study shows that otlertuzumab can be given at all doses explored with a tolerable toxicity profile. Notably, otlertuzumab demonstrated clinical activity as measured by standard response criteria, including reductions in lymphocyte counts and lymph node size and improvement in cytopenias. Response to otlertuzumab did vary partly on the basis of the amount of prior therapy administered. Responses to otlertuzumab occurred in 4 (16%) of 25 of patients with del(17p13). The high frequency of del(17p13) in patients in this trial raises the possibility that more activity would have been observed had a lower-genomic-risk patient population been studied. Otlertuzumab demonstrated dose-dependent PK, with no demonstrated correlation of Cmax or AUC to response in this study. While multiple schedules were pursued, a weekly schedule of otlertuzumab administration above 3 mg/kg generated a detectable Cmin prior to the following dose at concentrations shown to produce in vitro apoptosis and ADCC. Collectively, these data provide support for the feasibility of developing otlertuzumab as a therapeutic in CLL.
Toxicity noted in this study with otlertuzumab was similar to that reported with other B-cell–depleting therapeutics that depend in part upon the mechanism of ADCC. Specifically, 29% of patients experienced some infusion-related events during the first administration of otlertuzumab that usually did not recur with subsequent treatment doses. Only two patients had severe infusion reactions with otlertuzumab that required cessation of treatment. Hypophosphatemia was observed but the etiology was unclear. This laboratory abnormality was transient and clinically asymptomatic but deserves monitoring in future trials. While immune reaction to otlertuzumab in terms of antibody production was rare, it did occur in 4 individuals on this study. Toxicity was not apparent in any of these individuals with respect to serum sickness or increasing infusion events; moreover, titers were only significant in one patient. Development of ADA with any modified antibody structure is potentially expected, but in the case of otlertuzumab thus far, ADA development does not appear to raise significant concerns.
The single agent activity of otlertuzumab is modest, and in light of the high overall response rates with single-agent ibrutinib and idelalisib, it is reasonable to question the future role for otlertuzumab in the treatment of CLL. However, despite the robust results with the newer agents, CLL remains incurable. The ability to administer otlertuzumab for an extended period of time with clinical efficacy suggests that this agent could be integrated into existing and future therapies for CLL. Preclinical data demonstrating additive or synergistic activity in vitro and in vivo with rituximab and bendamustine have been reported, providing a rationale for evaluating each of these combinations.19,20,26 Additionally, the recent report demonstrating that CD37 ligation by otlertuzumab promotes death and survival signals via ITIM-mediated SHP1 activation and ITAM-like PI3-kinase δ activation provides impetus for combination with PI3-kinase inhibitors such as GS-1101.22 Indeed, preclinical in vitro data support this combination.22 Clinical trials of the combination of otlertuzumab with either bendamustine or rituximab are ongoing and will help define the utility of this molecule.
Portions of this study were presented at the 51st American Society of Hematology Annual Meeting and Exposition, New Orleans, LA, December 5-8, 2009; the 52nd Annual Meeting, Orlando, FL, December 4-7, 2010; and the 53rd Annual Meeting, San Diego, CA, December 10-13, 2011.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors wish to thank the patients who participated in this trial and their families who supported them. Additionally, we wish to thank the subinvestigators and research staff at each site who made completion of this trial possible.
This work supported by the National Cancer Institute (R01 CA159296 and P01 CA095426), the Leukemia & Lymphoma Society, and the D. Warren Brown Foundation.
Authorship
Contribution: J.C.B. planned and wrote the clinical trial, oversaw the work, wrote the first draft of the paper, and revised and approved the final manuscript; J.M.P., F.T.A., A.F., I.W.F., D.P.D.-L., S.E.S., L.A.A., A.K.G., J.P.L., and R.R.F. helped plan the trial, enroll patients, review drafts of the paper and approve the final version; A.J.E., J.E.B., and S.C.S. helped plan the trial and write about it, assist in amendments, oversee performance of the study, participate in data interpretation and summation, revise the manuscript and approve the final manuscript.
Conflict-of-interest disclosure: A.J.E., J.E.B., and S.C.S. have financial interests in otlertuzumab and are employees of Emergent Biosciences. The remaining authors declare no competing financial interests.
Correspondence: John C. Byrd, 455 OSUCCC Bldg, 410 West 12th Ave, Columbus, OH 43210; e-mail: john.byrd@osumc.edu.