In this issue of Blood, McIver et al demonstrate that components of the exosome complex are endogenous suppressors of erythroid maturation, revealing a new and novel regulatory mechanism for erythroid development.1
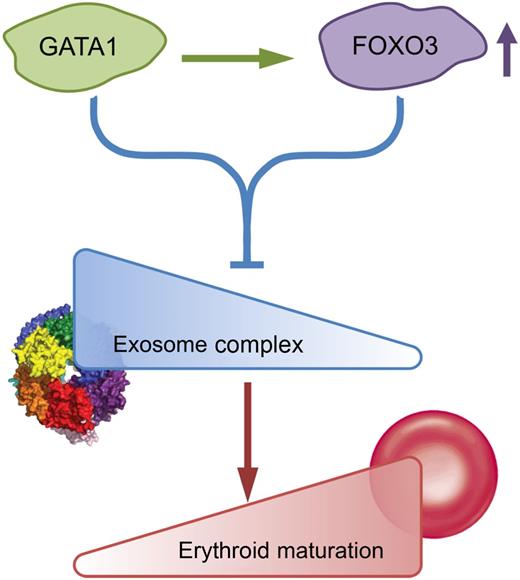
Schematic model of GATA1/Foxo3-exosome complex network. Exosome complex components create an erythroid barricade at the early stage of erythropoiesis. During the process of GATA1/Foxo3-induced erythroid maturation, GATA1 directly upregulates Foxo3 and functions together to repress the expression of exosome complex components, thus abrogating the blockade and allowing the erythroid maturation to proceed. The figure has been adapted from Figure 7 in the article by McIver et al that begins on page 2285.
Schematic model of GATA1/Foxo3-exosome complex network. Exosome complex components create an erythroid barricade at the early stage of erythropoiesis. During the process of GATA1/Foxo3-induced erythroid maturation, GATA1 directly upregulates Foxo3 and functions together to repress the expression of exosome complex components, thus abrogating the blockade and allowing the erythroid maturation to proceed. The figure has been adapted from Figure 7 in the article by McIver et al that begins on page 2285.
Erythropoiesis is a process during which pluripotent hematopoietic stem cells proliferate, differentiate, and eventually form mature erythrocytes. Eight distinct developmental stages including burst-forming unit-erythroid, colony-forming unit-erythroid, proerythroblast, basophilic erythroblast, polychromatic erythroblast, orthochromatic erythroblast, reticulocyte, and mature erythrocyte can be distinguished during this process, which is accompanied by many characteristic changes including decrease in cell size, chromatin condensation, enucleation, and increase in hemoglobin synthesis. Because of the uniqueness of cellular features that accompany erythroid differentiation, as well as clinical significance of erythropoiesis, the physiologic basis for the regulation of normal and disordered erythropoiesis in humans and in animals has been studied extensively. However, the primary focus of these studies has been on the roles of cytokines and transcription factors on the regulation of erythropoiesis. To date, the most extensively studied regulators include erythropoietin, erythropoietin receptor, and the transcription factors GATA1 and KLF1, all of which play critical roles in regulating erythroid development. In contrast, much less is known about the regulation of erythropoiesis by other mechanisms.
It is well documented that GATA1, KLF1, and other transcription factors such as Foxo3 control erythropoiesis by regulating the expression of a large number of erythroid-specific genes through modulation of sophisticated genetic networks. However, the way in which different transcription factors interact with each other to establish these genetic networks remains largely unexplored. In this regard, the authors’ group has recently demonstrated that GATA1 directly upregulates Foxo3 expression, which in turn amplifies GATA1 activity to regulate expression of genes involved in autophagy.2 In the present study, the authors further explore the scope of GATA1/Foxo3 cooperativity. By analyzing the GATA1/Foxo3-dependent transcriptome in erythroid cells, they found that GATA1/Foxo3 coregulate a cohort of genes in erythroid cells beyond the previously identified autophagy genes. Interestingly, the top categories in the corepressed cohort of genes are the ones involved in “noncoding RNA metabolic process” or “RNA processing.” Specifically, two of the highly GATA1/Foxo3-corepressed genes are Exosc8 and Exosc5, pivotal components of exosome complex. Importantly, knockdown of Exosc8 or other 2 exosome complex components induced erythroid maturation, demonstrating a role for exosome complex in suppressing erythroid development. A schematic model of this network is shown in the figure. In this network, exosome complex components create a barrier for erythroid differentiation at the early stage of erythropoiesis.
Mechanistically, the authors showed that downregulating Exosc8 enhanced expression of several GATA1-activated genes including Hbb-b1, Alas2, and Slc4a1. Based on these findings, they suggest that GATA1-mediated repression of Exosc8 during erythropoiesis contributes to the expression of GATA1 target genes important for erythroid maturation. They also showed that Exosc8 knockdown induced cell-cycle exit and upregulated the expression of several cell-cycle genes that are involved in cell-cycle arrest. Given the fact that both erythroid-specific genes and cell-cycle regulatory genes are suppressed by the exosome complex, the maturation blockade created by the exosome complex components probably involves multiple mechanisms.
Exosome complex is a multiprotein complex consisting of 9 core subunits and 3 catalytic subunits and was originally discovered as RNA elimination machinery.3 Later studies demonstrated that it is also involved in a wide variety of other molecular processes such as regulation of transcription start usage, maintenance of a heterochromatin marker, and regulation of noncoding RNA expression. The study by Mclver and colleagues provides convincing evidence of a prominent role for exosome complex in the biological process of erythroid development. Although the authors demonstrated that Exosc8 regulates a cohort of GATA1 target genes and a set of “cell-cycle arrest” genes, the mechanisms for this need to be further explored.
This study is important in several ways. First, the authors used a novel approach to analyze the genetic networks coregulated by GATA1 and Foxo3. This approach enabled them to identify a category of genes that would otherwise not be predictable based on analyses of GATA1 or Foxo3 function individually. Second, the linking of balanced exosome integrity to the onset of erythroid differentiation is a novel concept. In contrast to many previously identified genes that promote erythroid development, this is probably the first identification of factors that function as endogenous suppressors of the erythroid development program. Furthermore, it is interesting to note that this blockade is abrogated by GATA1 and Foxo3, which function in concert to maximally repress the expression of exosome complex components. Considering the complexity of erythroid development regulatory networks, it is reasonable to speculate that other endogenous or exogenous suppressors of erythroid developmental program may exist. Identification of these additional factors and how they orchestrate with other pathways should further our understanding of the regulation of erythropoiesis.
What are the clinical implications of these studies? Clinically, there are many cases of anemia associated with altered erythropoiesis where the causative mutations are still unknown. Interestingly, it has been shown that mutations in exosome complex components Exosc3 and Exosc8 are associated with human neurologic diseases.4,5 It will be interesting to examine whether the mutations of exosome components contributes to disordered erythropoiesis in inherited and acquired hematologic disease. However, in light of the growing evidence demonstrating the significant differences between human and murine erythropoiesis,6,7 it will be important to demonstrate whether the exosome complex components identified in regulating murine erythropoiesis play similar roles during human erythroid development.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal