Key Points
Early-differentiated NK cells accumulate and proliferate during IM.
These early-differentiated NK cells preferentially target lytic EBV-infected B cells in vitro.
Abstract
A growing body of evidence suggests that the human natural killer (NK)-cell compartment is phenotypically and functionally heterogeneous and is composed of several differentiation stages. Moreover, NK-cell subsets have been shown to exhibit adaptive immune features during herpes virus infection in experimental mice and to expand preferentially during viral infections in humans. However, both phenotype and role of NK cells during acute symptomatic Epstein-Barr virus (EBV) infection, termed infectious mononucleosis (IM), remain unclear. Here, we longitudinally assessed the kinetics, the differentiation, and the proliferation of subsets of NK cells in pediatric IM patients. Our results indicate that acute IM is characterized by the preferential proliferation of early-differentiated CD56dim NKG2A+ immunoglobulin-like receptor- NK cells. Moreover, this NK-cell subset exhibits features of terminal differentiation and persists at higher frequency during at least the first 6 months after acute IM. Finally, we demonstrate that this NK-cell subset preferentially degranulates and proliferates on exposure to EBV-infected B cells expressing lytic antigens. Thus, early-differentiated NK cells might play a key role in the immune control of primary infection with this persistent tumor-associated virus.
Introduction
Natural killer (NK) cells are a subset of innate lymphocytes that exhibit nonredundant antiviral functions in experimental mice.1 In mice infected with the murine cytomegalovirus (MCMV), a subset of NK cells bearing the activating receptor Ly49H expands and persists at increased frequency for more than 2 months following primary infection. Notably, these cells display an enhanced protective response against MCMV in adoptive transfer experiments.2 In humans, the peripheral blood compartment of NK cells is heterogeneous and accounts for 5% to 15% of lymphocytes. It is composed of diverse differentiation stages, which can be defined by the expression of surface markers, such as the 2 types of inhibitory receptors NKG2A and killer-cell immunoglobulin-like receptors (KIRs).3,4 Human NK cells seem to play an important antiviral role, because patients with isolated NK-cell deficiencies exhibit an increased susceptibility to herpes viruses.5 Furthermore, patients with acute viral infections resulting from hantavirus, cytomegalovirus (CMV), or chikungunya virus6-8 accumulate the late-differentiated CD56dim NKG2C+ KIR+ NK-cell subset in peripheral blood. However, none of these previous studies demonstrated a protective role for specifically accumulated human NK-cell subsets against virus-infected cells in vitro or in vivo.9,10
A ubiquitous persistent human virus, which has not been investigated in detail in this respect, is the primarily B-cell-tropic Epstein-Barr virus (EBV). EBV is a γ-herpes virus, which latently infects the vast majority of the adult human population worldwide, and is associated with B-cell and epithelial-cell malignancies.11 EBV displays 2 modes of infection. One mode expresses latency genes (latent EBV) leading to B-cell transformation in vitro and subsequent generation of lymphoblastoid cell lines (LCLs). The other mode expresses lytic genes (lytic EBV) leading to the production of infectious viral particles and lysis of the host cell.12 Most primary EBV infections occur before the age of 5 years and are usually asymptomatic. Nevertheless, primary EBV infection occurring beyond this age may manifest as infectious mononucleosis (IM) that affects around 10% of the population in Europe and the United States.13,14 The usually self-limiting IM is characterized by a vigorous CD8+ T-cell response that mainly targets EBV lytic epitopes15 and is associated with an increased risk of developing EBV-positive classic Hodgkin lymphoma.16
The contribution of particular NK-cell subsets to the immune control of EBV, especially during primary infection, remains elusive. Here, we examined how blood NK-cell subsets accumulate and respond during IM, and to what extent they can recognize latently and lytically EBV-infected B cells.
Material and methods
Study design
Twenty-two pediatric patients diagnosed with acute IM at the University Children’s Hospital of Zurich were prospectively enrolled between October 2010 and April 2013. The onset date of symptoms was used as reference for the longitudinal study. Twelve pediatric patients with IM symptoms, but lacking the serological pattern compatible with acute EBV infection, were also enrolled (IM-like) and donated peripheral blood at diagnosis. All serum samples from IM-like patients were negative for HCMV DNA. Healthy children and healthy adults aged 20 to 30 years were used as healthy controls according to their EBV serology. Further details are outlined in the supplemental Methods available on the Blood Web site.
All participants provided informed consent in accordance with the Declaration of Helsinki, and the institutional ethics committee approved all protocols used.
Monoclonal antibodies and flow cytometry
Samples were acquired on a FACSCanto II and an LSR Fortessa (BD Biosciences). Details about the handling of PBMCs, flow cytometry analysis, and antibodies used are described in the supplemental Methods.
Cell lines
Preparation of viral stocks, cell lines used, and induction and isolation of lytic AKBM cells as well as the degranulation assay are described in the supplemental Methods.
Viral loads quantification
EBV DNA levels were determined by real-time polymerase chain reaction. The details of viral load measurements are outlined in the supplemental Methods.
Statistical analysis
Data were analyzed using Prism software (GraphPad Software, Inc.). P values of <.05 were considered significant and were calculated with the nonparametric Mann-Whitney U test or the Wilcoxon matched-pairs signed ranks tests. Spearman’s rank correlation was used to examine associations between 2 quantitative values.
Results
Pediatric acute IM patients exhibit accumulation of activated CD8+ T cells and CD56dim NK cells
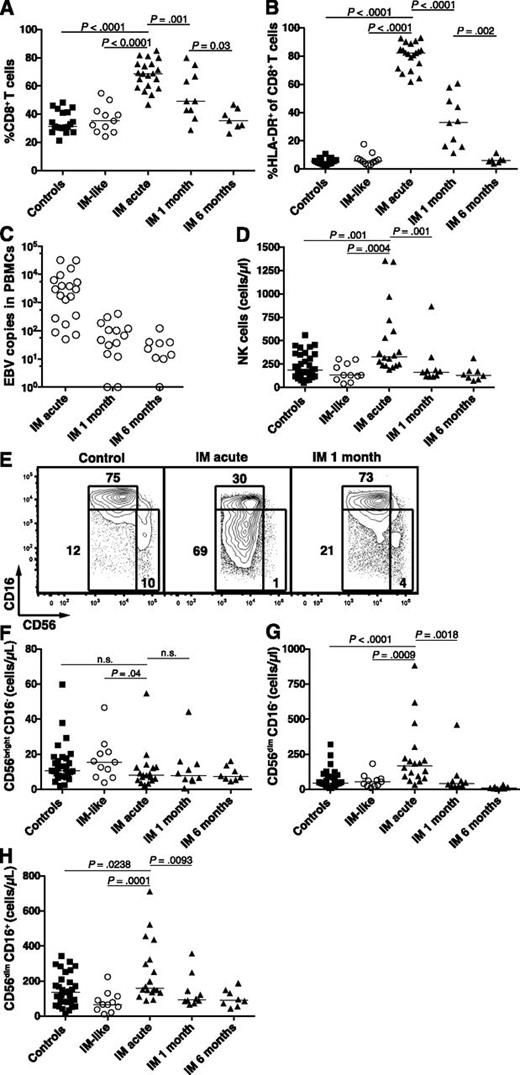
In young adults, IM is characterized by vigorous T-cell responses mediated mainly by EBV-specific CD8+ T cells.15 Nevertheless, neither T-cell nor NK-cell responses in pediatric IM have been characterized. Because we examined pediatric IM patients only, we first assessed the dynamics of the T-cell and NK-cell subset responses in pediatric patients during the first 6 months of IM. Uninfected healthy individuals and pediatric patients with IM-like diseases were used as controls. Acute IM patients exhibited a twofold increased median frequency of CD8+ T cells (Figure 1A), a 15-fold increased median frequency of activated CD8+ T cells (Figure 1B), and 60-fold increased median HLA-DR+ CD8+ T-cell counts (data not shown) compared with controls. The numbers of activated T cells normalized within 6 months. These changes paralleled those of the cellular EBV DNA levels through time (Figure 1C). Neither EBV DNA levels nor frequency of CD8+ T cells correlated with age (supplemental Figure 1). Therefore, pediatric IM patients as young as 2 years of age seem to exhibit the classic immunologic features found in young adults with IM.
Accumulation of activated HLA-DR+ CD8+ T cells and CD56dim NK cells during acute IM. PBMCs from healthy controls, IM-like patients, and IM patients at acute phase (IM acute), at 1 (IM 1 month), and 6 months (IM 6 months) were analyzed by flow cytometry. Frequencies of (A) CD8+ T cells within the CD3+ T-cell population and (B) HLA-DR+ CD8+ T cells within the CD8+ T-cell population in healthy controls (n = 19), IM-like (n = 11), and IM acute (n = 20), 1-month (n = 10) and 6-month (n = 7) patients. (C) EBV DNA load in copies per 106 PBMCs in IM-acute (n = 19), 1-month (n = 14), and 6-month (n = 9) patients. Counts (cells/µL blood) of total NK cells (D) and frequencies of CD56bright CD16−, CD56dim CD16−, and CD56dim CD16+ NK-cell subsets within the CD3− CD56+ NK-cell population from representative healthy control, IM-acute and 1-month patient (E). Counts (cells/µL blood) of (F) CD56bright CD16−, (G) CD56dim CD16−, and (H) CD56dim CD16+ NK cells in healthy controls (n = 31), IM-like patients (n = 11), and IM-acute (n = 18), 1-month (n = 10), and 6-month (n = 8) patients.
Accumulation of activated HLA-DR+ CD8+ T cells and CD56dim NK cells during acute IM. PBMCs from healthy controls, IM-like patients, and IM patients at acute phase (IM acute), at 1 (IM 1 month), and 6 months (IM 6 months) were analyzed by flow cytometry. Frequencies of (A) CD8+ T cells within the CD3+ T-cell population and (B) HLA-DR+ CD8+ T cells within the CD8+ T-cell population in healthy controls (n = 19), IM-like (n = 11), and IM acute (n = 20), 1-month (n = 10) and 6-month (n = 7) patients. (C) EBV DNA load in copies per 106 PBMCs in IM-acute (n = 19), 1-month (n = 14), and 6-month (n = 9) patients. Counts (cells/µL blood) of total NK cells (D) and frequencies of CD56bright CD16−, CD56dim CD16−, and CD56dim CD16+ NK-cell subsets within the CD3− CD56+ NK-cell population from representative healthy control, IM-acute and 1-month patient (E). Counts (cells/µL blood) of (F) CD56bright CD16−, (G) CD56dim CD16−, and (H) CD56dim CD16+ NK cells in healthy controls (n = 31), IM-like patients (n = 11), and IM-acute (n = 18), 1-month (n = 10), and 6-month (n = 8) patients.
Moreover, we observed a 1.7-fold increase in the median numbers of NK cells in acute IM compared with controls. These numbers returned to baseline levels after 1 month (Figure 1D). The blood NK-cell compartment is mainly composed of 2 well-characterized functional subsets, the CD56bright CD16− and the CD56dim CD16+ subsets.17 The former NK-cell subset produces large amounts of cytokines on monokine stimulation, acquires cytotoxicity only after prolonged activation, and is enriched in secondary lymphoid organs.18 The latter NK-cell subset readily kills susceptible targets and can rapidly secrete IFN-γ on engagement of activating receptors.19 Acute IM patients displayed unchanged counts of CD56bright CD16− NK cells (Figure 1F) and a 1.2-fold increase in the median count of CD56dim CD16+ NK cells (Figure 1H) compared with controls. Interestingly, the intermediate NK-cell subset CD56dimCD16− was increased in frequency during acute IM (Figure 1E and supplemental Figure 2A) and exhibited a 3.7-fold increase in median cell counts compared with controls (Figure 1G). Thus we found a selective increase of the total CD56dim NK-cell subset during acute IM.
Early-differentiated CD56dim NKG2A+ KIR− NK cells accumulate during IM and terminally differentiate as well as persist afterward
To dissect the CD56dim NK-cell subset, we analyzed the expression patterns of the inhibitory receptors NKG2A and KIRs, which might allow assessment of subtle maturation stages spanning from early-differentiated CD56dim NKG2A+ KIR− to late-differentiated CD56dim NKG2A− KIR+ NK cells.3,4 Acute IM patients exhibited a 1.8-fold increased median frequency of CD56dim NKG2A+ KIR− NK cells (Figure 2A-B), but a 1.5-fold reduced median frequency of CD56dim NKG2A− KIR+ NK cells compared with EBV-negative and EBV-positive control individuals (Figure 2C). Moreover, acute IM patients displayed 4.8-fold and 3.5-fold increased median absolute numbers of CD56dim NKG2A+ KIR− NK cells compared with EBV-negative and EBV-positive control individuals, respectively (supplemental Figure 2B), but unchanged cell counts of the late-differentiated CD56dim NKG2A− KIR+ NK cells. We observed no major changes in the frequencies and cell counts of the CD56dim NKG2A− KIR− and CD56dim NKG2A+ KIR+ NK-cell subsets (supplemental Figure 2B-C). Furthermore, IM-like patients, that is, patients with IM symptoms but no acute EBV infection, did not exhibit NK-cell subset accumulation similar to that of IM patients. We did not find any differences in the frequency of this NK-cell subset between EBV-seronegative and EBV-seropositive control individuals. Surprisingly, the frequency of CD56dim NKG2A+ KIR− NK cells remained significantly elevated in longitudinally followed IM patients up to 6 months after acute IM, but returned to baseline after 2 years (Figure 2B).
Accumulation and terminal differentiation of the CD56dim NKG2A+ KIR− NK-cell subset during acute IM. PBMCs from controls and IM patients were analyzed by flow cytometry. (A) Frequencies of CD56dim NKG2A+ KIR− NK cells within the CD56dim population from representative healthy control, IM-acute, 1-month, and 6-month patients. Frequencies of (B) CD56dim NKG2A+ KIR− and (C) CD56dim NKG2A− KIR+ NK cells in healthy EBV-negative controls (n = 17), healthy EBV-positive controls (n = 20), IM-like patients (n = 12), and IM-acute (n = 17), 1-month (n = 11), 6-month (n = 10), and 2-year (n = 4) patients. Frequencies of CD57+ cells within (D) the CD56dim NKG2A+ KIR− and (E) the CD56dim NKG2A− KIR+ NK-cell subsets in healthy EBV-negative controls (n = 17), healthy EBV-positive controls (n = 20), and IM-acute (n = 17), 1-month (n = 11), 6-month (n = 10), and 2-year (n = 4) patients. Horizontal lines or single symbols indicate median values. Error bars indicate interquartile ranges. Mann-Whitney U tests.
Accumulation and terminal differentiation of the CD56dim NKG2A+ KIR− NK-cell subset during acute IM. PBMCs from controls and IM patients were analyzed by flow cytometry. (A) Frequencies of CD56dim NKG2A+ KIR− NK cells within the CD56dim population from representative healthy control, IM-acute, 1-month, and 6-month patients. Frequencies of (B) CD56dim NKG2A+ KIR− and (C) CD56dim NKG2A− KIR+ NK cells in healthy EBV-negative controls (n = 17), healthy EBV-positive controls (n = 20), IM-like patients (n = 12), and IM-acute (n = 17), 1-month (n = 11), 6-month (n = 10), and 2-year (n = 4) patients. Frequencies of CD57+ cells within (D) the CD56dim NKG2A+ KIR− and (E) the CD56dim NKG2A− KIR+ NK-cell subsets in healthy EBV-negative controls (n = 17), healthy EBV-positive controls (n = 20), and IM-acute (n = 17), 1-month (n = 11), 6-month (n = 10), and 2-year (n = 4) patients. Horizontal lines or single symbols indicate median values. Error bars indicate interquartile ranges. Mann-Whitney U tests.
CD57 has been proposed as a marker of terminal differentiation of NK cells3,20 and has been shown to be upregulated on CD56dim NKG2C+ NK cells during acute infection with CMV, hantavirus, or chikungunya virus.6-8 Therefore, we hypothesized that if the accumulated CD56dim NKG2A+ KIR− subset found in acute IM patients is preferentially involved in the immune response against EBV, it should acquire CD57 during the acute phase of IM to complete its terminal differentiation. Indeed, we observed a 2.5-fold increase in the median frequency of CD57+ within the CD56dim NKG2A+ KIR- subset from the acute IM phase to 1 month later, but no changes in the CD56dim NKG2A− KIR+ subset (Figure 2D-E, respectively). Thus, acute symptomatic EBV infection elicits the specific accumulation of the CD56dim NKG2A+ KIR- NK-cell subset, its terminal differentiation, as well as its persistence at higher frequency during the first 6 months after acute IM.
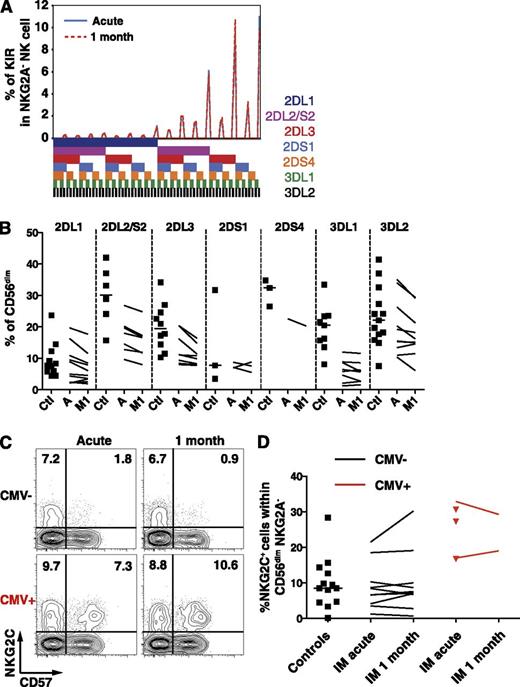
IM patients exhibit a stable KIR repertoire and unchanged frequencies of CD56dim NKG2C+ NK cells
The KIR repertoire is composed of several activating and inhibitory receptors specific for distinct groups of HLA class I alleles,21 is highly variable among individuals, and is stable throughout time in healthy adults.22 Particular KIR receptors might be involved in the immune control of viruses such as HIV,23-26 the hepatitis C virus,27,28 or CMV.22,29 Therefore, to ensure that IM does not lead to an accumulation of NK cells bearing specific KIRs, we performed a comprehensive phenotypic KIR analysis30 in a group of CMV-seronegative healthy controls and IM patients. The KIR repertoire of IM patients remained stable throughout the first month of IM (Figure 3A), and the frequencies of single KIR+ CD56dim NK cells in IM patients were overall low compared with controls (Figure 3B). Thus, IM is associated with the accumulation of a CD56dim NKG2A+ NK-cell subset, which does not carry any increase in activating, nor inhibitory, KIR molecule expression. Furthermore, CD56dim NK cells expressing NKG2C, the activating counterpart of NKG2A,31 accumulate on CMV infection,7,32,33 as well as on other viral infections in CMV-positive individuals.6,8,34-36 Thus, we assessed whether a similar accumulation occurs on EBV infection and therefore investigated CMV-seronegative IM patients and controls to avoid bias associated with CMV carriage.37 We observed no changes in the frequency of NKG2C+ NK cells within the CD56dim NKG2A− NK-cell subset (Figure 3C-D) nor within the expanding CD56dim NKG2A+ KIR− NK-cell subset (data not shown). Thus, CD56dim NKG2A+ KIR− NKG2C− NK cells accumulate during IM.
Frequencies of single KIR-positive and NKG2C+ CD56dim NK cells are not altered during acute IM. (A) Frequencies of CD56dim NKG2A− NK cells expressing the 7 analyzed KIRs from 1 representative CMV-seronegative IM patient at acute phase and at 1 month. The presence of 1 KIR in a combination is represented by a color code below the graph. (B) Frequencies of single KIR-positive CD56dim NK cells in healthy controls (Ctl, n = 11) and IM patients (n = 10) at acute phase (A) and at 1 month (M1). (C) Frequencies of NKG2C+ CD57+ NK cells within the CD56dim NKG2A− population from 1 CMV-seronegative and 1 CMV-seropositive IM patient at acute phase and at 1 month. (D) Frequencies of NKG2C+ NK cells within the CD56dim NKG2A− NK cell population in CMV-seronegative healthy controls (n = 13), CMV-seronegative (n = 10), and CMV-seropositive (n = 5, red) IM patients at acute phase and at 1 month (n = 2 for CMV-seropositive, red lines).
Frequencies of single KIR-positive and NKG2C+ CD56dim NK cells are not altered during acute IM. (A) Frequencies of CD56dim NKG2A− NK cells expressing the 7 analyzed KIRs from 1 representative CMV-seronegative IM patient at acute phase and at 1 month. The presence of 1 KIR in a combination is represented by a color code below the graph. (B) Frequencies of single KIR-positive CD56dim NK cells in healthy controls (Ctl, n = 11) and IM patients (n = 10) at acute phase (A) and at 1 month (M1). (C) Frequencies of NKG2C+ CD57+ NK cells within the CD56dim NKG2A− population from 1 CMV-seronegative and 1 CMV-seropositive IM patient at acute phase and at 1 month. (D) Frequencies of NKG2C+ NK cells within the CD56dim NKG2A− NK cell population in CMV-seronegative healthy controls (n = 13), CMV-seronegative (n = 10), and CMV-seropositive (n = 5, red) IM patients at acute phase and at 1 month (n = 2 for CMV-seropositive, red lines).
The preferential proliferation of CD56dim NKG2A+ KIR- NK cells positively correlates with cellular EBV loads during acute IM
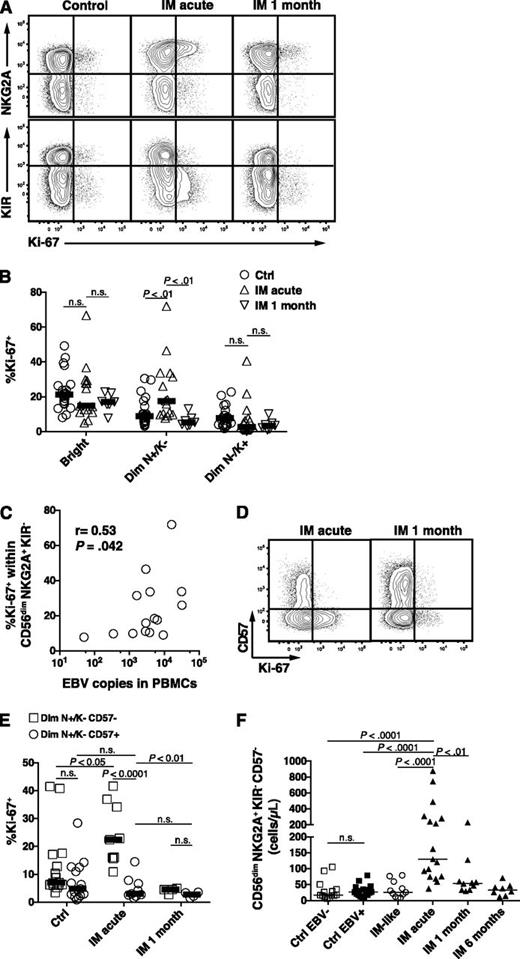
We next investigated whether the increase in the absolute numbers of CD56dim NKG2A+ KIR- NK cells might be caused by active proliferation of this NK-cell subset. We assessed the expression of the proliferation marker Ki-67 in the CD56bright, the CD56dim NKG2A+ KIR−, and the CD56dim NKG2A− KIR+ NK-cell subsets in acute IM 1 month later. We found a twofold and a threefold increase in the median frequency of Ki-67+ cells within the CD56dim NKG2A+ KIR− NK cells in acute IM compared with controls and IM at 1 month, respectively (Figure 4A-B). However, we did not observe any increased proliferation in the more differentiated NKG2A− KIR+ NK subset, and there was no difference when comparing EBV-negative with EBV-positive controls (Figure 4A-B and data not shown). In addition, the precursor CD56bright NK-cell subset, which exhibits strong proliferation properties and responds to minute doses of cytokines in vitro, did not show an increased frequency of Ki-67+ cells in acute IM patients (Figure 4B). Notably, the frequencies of proliferating Ki-67+ cells within the CD56dim NKG2A+ KIR- positively correlated with EBV DNA levels in PBMCs (Figure 4C), but not in serum (data not shown). No such correlation was observed within the CD56dim NKG2A− KIR+ NK-cell subset. This suggested that EBV-infected B cells in IM patients might directly drive the proliferation of early-differentiated CD56dim NK cells. We next asked whether the proliferation of the CD56dim NKG2A+ KIR− NK-cell subset differs according to CD57 expression. Surprisingly, proliferation was exclusively found within the CD57- fraction (Figure 4D-E). Our finding aligns with previous studies showing a decreased proliferative potential of CD57+ NK cells compared with CD57− NK cells.3,20 Thus, CD56dim NKG2A+ KIR− CD57− NK cells seem to proliferate preferentially during acute IM but not 1 month later. Moreover, in the acute phase, proliferation parallels the accumulation of this NK-cell subset, which displays a sevenfold and a 4.8-fold median increase compared with EBV-negative and EBV-positive controls, respectively (Figure 4F). In addition, CMV status did not seem to influence the NK-cell response (supplemental Figure 3). We did not observe any correlation between the count of the CD56dim NKG2A+ KIR− CD57− NK cells, nor the count of total NK cells, and the EBV DNA levels in PBMCs or in serum (data not shown). Thus, early-differentiated NK cells accumulate in IM patients after EBV-driven proliferation.
Increased count of CD56dim NKG2A+ KIR− CD57− NK cells during acute IM is caused by preferential proliferation. (A) Representative examples of costaining for NKG2A and Ki-67 and costaining for KIR and Ki-67 on CD56dim NK cells in healthy control and IM-acute and 1-month patients. (B) Frequencies of Ki-67+ cells within the CD56bright, CD56dim NKG2A+ KIR−, and CD56dim NKG2A− KIR+ NK-cell subsets in healthy controls (n = 21), in IM-acute (n = 15), and in 1-month patients (n = 7). (C) Correlation of EBV DNA loads (copies per 106 PBMCs) and frequencies of Ki-67+ cells within the CD56dim NKG2A+ KIR− NK-cell subset from acute IM patients. Spearman r = 0.53, P (2-tailed) = .042. (D) Representative example of costaining for CD57 and Ki-67 on CD56dim NKG2A+ KIR− NK cells in an IM-acute and 1-month patient. (E) Frequencies of Ki-67+ cells within the CD56dim NKG2A+ KIR− NK cells according to CD57 expression in healthy controls (n = 15), IM-acute (n = 9), and 1-month (n = 4) patients. (F) Count of CD56dim NKG2A+ KIR− CD57− in healthy EBV-negative controls (n = 14), healthy EBV-positive controls (n = 17), IM-like (n = 11), IM-acute (n = 17), 1-month (n = 10), and 6-month (n = 8) patients. Horizontal lines indicate median values of a given symbol. Mann-Whitney U tests.
Increased count of CD56dim NKG2A+ KIR− CD57− NK cells during acute IM is caused by preferential proliferation. (A) Representative examples of costaining for NKG2A and Ki-67 and costaining for KIR and Ki-67 on CD56dim NK cells in healthy control and IM-acute and 1-month patients. (B) Frequencies of Ki-67+ cells within the CD56bright, CD56dim NKG2A+ KIR−, and CD56dim NKG2A− KIR+ NK-cell subsets in healthy controls (n = 21), in IM-acute (n = 15), and in 1-month patients (n = 7). (C) Correlation of EBV DNA loads (copies per 106 PBMCs) and frequencies of Ki-67+ cells within the CD56dim NKG2A+ KIR− NK-cell subset from acute IM patients. Spearman r = 0.53, P (2-tailed) = .042. (D) Representative example of costaining for CD57 and Ki-67 on CD56dim NKG2A+ KIR− NK cells in an IM-acute and 1-month patient. (E) Frequencies of Ki-67+ cells within the CD56dim NKG2A+ KIR− NK cells according to CD57 expression in healthy controls (n = 15), IM-acute (n = 9), and 1-month (n = 4) patients. (F) Count of CD56dim NKG2A+ KIR− CD57− in healthy EBV-negative controls (n = 14), healthy EBV-positive controls (n = 17), IM-like (n = 11), IM-acute (n = 17), 1-month (n = 10), and 6-month (n = 8) patients. Horizontal lines indicate median values of a given symbol. Mann-Whitney U tests.
CD56dim NKG2A+ KIR− NK cells preferentially target EBV-infected B cells with lytic reactivation
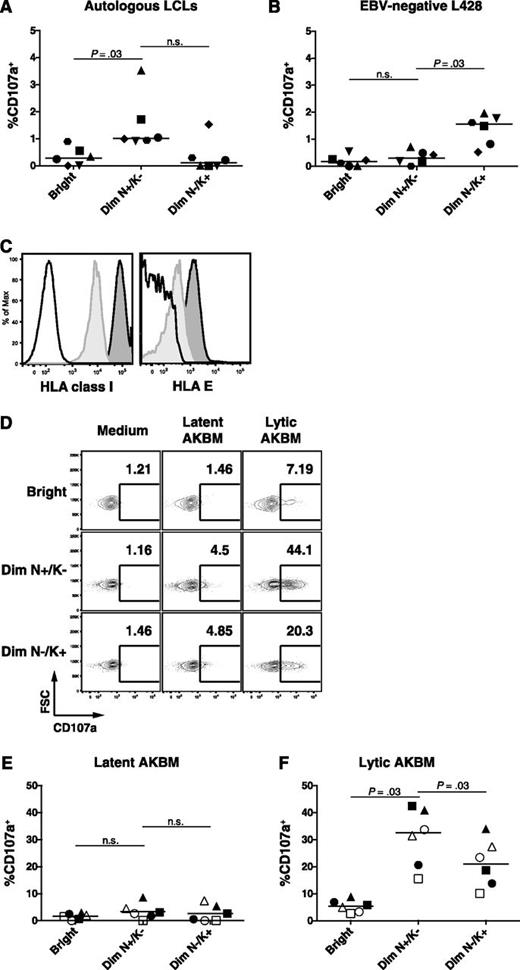
CD56dim NKG2A+ KIR− NK cells are functional against HLA-class-I-deficient target cells, including the EBV-positive LCL 721.221 cell line,4 but their reactivity toward HLA class I competent autologous LCLs has not yet been assessed. We observed a low overall frequency of degranulating NK cells on coculture with autologous LCLs. Nevertheless, the CD56dim NKG2A+ KIR− NK subset, which accumulates during IM, displayed a more than twofold increase in degranulation compared with the CD56bright and CD56dim NKG2A− KIR+ NK-cell subset (Figure 5A). In contrast, the EBV-negative allogeneic B-cell line L428, which exhibits an activated phenotype comparable to LCLs, elicits an increased response in the CD56dim NKG2A− KIR+ NK subset only (Figure 5B). This low NK-cell response against autologous LCLs might be a result of their high surface level of HLA class I and HLA-E which might engage the NK-cell inhibitory receptors KIR and NKG2A, respectively. Indeed, EBV-infected B cells upregulated HLA class I38 and HLA-E (Figure 5C).
Increased cytotoxic degranulation of the CD56dim NKG2A+ KIR− NK subset against EBV-infected B cells with lytic replication. (A) PBMCs from 6 healthy EBV-positive donors were cocultured with autologous LCLs and (B) EBV-negative L428 at an effector to target ratio of 10:1 for 6 hours. Frequencies of degranulating (CD107a+) cells within the CD56bright (Bright), the CD56dim NKG2A+ KIR− (Dim N+/K−), and the CD56dim NKG2A− KIR+ (Dim N−/K+) NK-cell subsets were assessed by flow cytometry at the end of the coculture. (C) HLA class I and HLA-E expression on CD19+ B cells from PBMCs (light gray histogram) and from autologous LCLs (dark gray histogram). Isotype controls are depicted as a white histogram. (D) Representative example of frequencies of CD107a+ NK cells within the 3 NK-cell subsets after coculture with latent AKBM or lytic AKBM. Frequencies of degranulating (CD107a+) NK cells within the CD56bright (Bright), the CD56dim NKG2A+ KIR− (Dim N+/K−) and the CD56dim NKG2A− matched KIR+ (Dim N−/K+) NK-cell subsets in PBMCs from 3 convalescent IM patients (open symbols) and 3 healthy EBV-positive donors (filled symbols) cocultured with (E) latent AKBM or (F) lytic AKBM (n = 6). Horizontal lines indicate median values of a given subset, Wilcoxon matched-pairs signed ranks tests.
Increased cytotoxic degranulation of the CD56dim NKG2A+ KIR− NK subset against EBV-infected B cells with lytic replication. (A) PBMCs from 6 healthy EBV-positive donors were cocultured with autologous LCLs and (B) EBV-negative L428 at an effector to target ratio of 10:1 for 6 hours. Frequencies of degranulating (CD107a+) cells within the CD56bright (Bright), the CD56dim NKG2A+ KIR− (Dim N+/K−), and the CD56dim NKG2A− KIR+ (Dim N−/K+) NK-cell subsets were assessed by flow cytometry at the end of the coculture. (C) HLA class I and HLA-E expression on CD19+ B cells from PBMCs (light gray histogram) and from autologous LCLs (dark gray histogram). Isotype controls are depicted as a white histogram. (D) Representative example of frequencies of CD107a+ NK cells within the 3 NK-cell subsets after coculture with latent AKBM or lytic AKBM. Frequencies of degranulating (CD107a+) NK cells within the CD56bright (Bright), the CD56dim NKG2A+ KIR− (Dim N+/K−) and the CD56dim NKG2A− matched KIR+ (Dim N−/K+) NK-cell subsets in PBMCs from 3 convalescent IM patients (open symbols) and 3 healthy EBV-positive donors (filled symbols) cocultured with (E) latent AKBM or (F) lytic AKBM (n = 6). Horizontal lines indicate median values of a given subset, Wilcoxon matched-pairs signed ranks tests.
On the other hand, induction of the lytic cycle of EBV infection has been shown to sensitize EBV-infected B cells to NK-cell killing using the EBV-positive Akata Burkitt lymphoma reporter cell line AKBM,39 which allows the purification of Akata Burkitt lymphoma cells with and without EBV lytic reactivation, respectively. Hence, we tested the degranulation of the CD56dim NKG2A+ KIR− NK subset against either latent AKBM or lytic AKBM cells from convalescent IM patients and healthy EBV-positive controls and compared it to the CD56bright and the CD56dim NKG2A− KIR+ subsets. To avoid HLA class I/KIR mismatch bias, we specifically assessed the degranulation in KIR+ matched (KIR2DL2/DL3/3DL1+) NK cells according to the AKBM genotype (Bw4/C1/C1). We could confirm increased responses of NK cells against lytic compared with latent AKBM cells (Figure 5D-F) previously shown to be mediated by a downregulation of the inhibitory ligands HLA-class I and HLA-E and an upregulation of the activating ligands CD112 and ULBP-1.39 Similarly, we also found increased expression of activating ligands on lytic EBV infected LCLs (supplemental Figure 4D-G), however the expression of the respective activating receptors in the CD56dim NKG2A+ KIR− NK-cell subset was unaltered in IM patients (supplemental Figure 4A-C). Importantly, the CD56dim NKG2A+ KIR− NK-cell subset exhibited a significantly stronger degranulation against lytic AKBM cells compared with all other subsets (Figure 5F). We found no difference in the degranulation capacity of CD56dim NKG2A+ KIR− NK between convalescent IM patients and controls, suggesting that these NK cells are functional and are not in an exhausted state in the aftermath of acute IM. Thus, CD56dim NKG2A+ KIR− NK cells preferentially recognize lytic EBV-replicating B cells.
EBV lytic replication triggers in vitro proliferation of NKG2A+ KIR- NK cells
Finally, we examined the proliferation of NKG2A+ KIR− NK cells using staining for Ki-6740 in an in vitro model of primary EBV infection of cord blood mononuclear cells (CBMC) infected with either wild-type (WT) EBV or lytic replication incompetent BZLF1-KO (BZ1KO) EBV. Both viruses elicited similar IFN-type I responses 24 hours postinfection (Figure 6A) and exhibited comparable infection capacity, as evaluated by the frequencies of GFP+ EBV-infected B cells 3 days postinfection (Figure 6B). Because most NK cells upregulated CD56 surface expression during in vitro culture (Figure 6C, first row), we did not distinguish between the CD56bright and CD56dim NK cells for further analysis. We observed an increased proliferation of NKG2A+ KIR− NK cells 7 days after infection with WT EBV compared with mock (Figure 6C-D). Infection with BZ1KO EBV, with abolished expression of all EBV lytic antigens, elicited a twofold-reduced proliferation of NKG2A+ KIR− NK cells, in comparison with WT EBV (median ratio 2.9 vs 6.3, P = .04). This indicates that the proliferation of NKG2A+ KIR− NK cells partially depends on the presence of EBV-infected cells expressing lytic antigens. Notably, NKG2A− KIR+ NK cells exhibit similar proliferation on infection with WT EBV, but this is not affected by the absence of lytic antigens (data not shown). Finally, we assessed a possible age-dependent distribution of this early-differentiated NK-cell subset in peripheral blood from healthy individuals that might correlate with the known age-dependent prevalence of primary symptomatic EBV infection. Indeed, CD56dim NKG2A+ KIR− NK cells both decreased in frequency (Figure 6E) and in absolute numbers (Figure 6F) during the first decade of life, whereas the counts of CD56dim NKG2A− KIR+ NK cells remained unchanged with age (data not shown). Thus, CD56dim NKG2A+ KIR− NK cells, which decrease in frequency in the first decade of life, preferentially degranulate and proliferate in response to lytic EBV-replicating B cells.
EBV-driven in vitro proliferation of NKG2A+ KIR- NK cells partially depends on expression of lytic antigens. Proliferation of NK-cell subsets was assessed 7 days after inoculation of CBMCs with either WT EBV, BZLF1-KO (BZ1KO) EBV or PBS (MOCK). (A) Concentrations of IFN-α in supernatant 24-hour postinoculation on MOCK, WT EBV, and BZ1KO EBV infection (pg/mL; n = 5). (B) Representative example of CD19 and GFP costaining within live lymphocytes 72 hours postinfection. Numbers indicate frequencies of GFP-negative or GFP-positive cells within the CD19+ B-cell population. (C) Frequencies of Ki-67+ NKG2A+ KIR− NK cells 7 days after inoculation of CBMCs with mock, WT EBV, or BZ1KO EBV, or after stimulation with IL-2. The depicted gates were assessed within live lymphocytes (first row), CD3− CD56+ NK cells (second row), and NKG2A+ KIR− NK cells (third row). (D) Ratio of NKG2A+ KIR− Ki-67+ NK-cell counts from WT EBV- or BZ1KO EBV- over mock-infected CBMCs. (E) Frequencies of CD56dim NKG2A+ KIR− NK cells in newborns (n = 8), children aged 1 to 5 years (n = 16), children aged 5 to 15 years (n = 17), and adults aged 20 to 30 years (n = 15). (F) Counts of CD56dim NKG2A+ KIR- NK cells in children aged 1 to 5 years (n = 14), children aged 5 to 15 years (n = 15), and adults aged 20 to 30 years (n = 15). Horizontal lines indicate median values of a given age group, Mann-Whitney U tests.
EBV-driven in vitro proliferation of NKG2A+ KIR- NK cells partially depends on expression of lytic antigens. Proliferation of NK-cell subsets was assessed 7 days after inoculation of CBMCs with either WT EBV, BZLF1-KO (BZ1KO) EBV or PBS (MOCK). (A) Concentrations of IFN-α in supernatant 24-hour postinoculation on MOCK, WT EBV, and BZ1KO EBV infection (pg/mL; n = 5). (B) Representative example of CD19 and GFP costaining within live lymphocytes 72 hours postinfection. Numbers indicate frequencies of GFP-negative or GFP-positive cells within the CD19+ B-cell population. (C) Frequencies of Ki-67+ NKG2A+ KIR− NK cells 7 days after inoculation of CBMCs with mock, WT EBV, or BZ1KO EBV, or after stimulation with IL-2. The depicted gates were assessed within live lymphocytes (first row), CD3− CD56+ NK cells (second row), and NKG2A+ KIR− NK cells (third row). (D) Ratio of NKG2A+ KIR− Ki-67+ NK-cell counts from WT EBV- or BZ1KO EBV- over mock-infected CBMCs. (E) Frequencies of CD56dim NKG2A+ KIR− NK cells in newborns (n = 8), children aged 1 to 5 years (n = 16), children aged 5 to 15 years (n = 17), and adults aged 20 to 30 years (n = 15). (F) Counts of CD56dim NKG2A+ KIR- NK cells in children aged 1 to 5 years (n = 14), children aged 5 to 15 years (n = 15), and adults aged 20 to 30 years (n = 15). Horizontal lines indicate median values of a given age group, Mann-Whitney U tests.
Discussion
Here we demonstrate in longitudinally followed pediatric IM patients that an early-differentiated CD56dim NKG2A+ KIR− NK subset selectively accumulates during primary symptomatic EBV infection and persists at increased frequencies for months. Moreover, our data indicate that these NK cells specifically recognize B cells undergoing lytic EBV replication. Our findings are unprecedented and suggest that responses of NK-cell subsets to viral infections may not be confined to late-differentiated populations.6-8 Moreover, distinct NK-cell subsets may be rather pathogen-specific.
Remarkably, although we found increased counts in the cytotoxic CD56dim NK-cell subset, but not in the less-differentiated CD56bright CD16- NK-cell subset, we could not confirm the previously reported expansion of CD56bright CD16+ NK cells during acute symptomatic EBV infection.41 We rather observed an unusual increase of the intermediate CD56dim CD16− NK-cell subset, which might be explained by downregulation of CD56 on CD56bright NK cells or by downregulation of CD16 such as observed in degranulating CD56dim NK cells on coculture with K562 cells (unpublished observations). This CD56dim NK-cell subset is distinctly characterized by NKG2A expression and by the absence of KIRs. It strikingly differs from that of other acute viral infections such as CMV, hantavirus, or chikungunya virus infection,6-8 in which the CD56dim KIR+ NKG2C+ NK-cell subset was shown to be expanded. CD56dim NKG2A+ KIR- NK cells are considered early-differentiated4 as suggested by the specific temporal reconstitution of the NK-cell subsets in hematopoietic stem-cell transplanted patients42-44 and in mice with human immune-system components.3 Based on our results, ongoing differentiation of these early-differentiated NK cells seems to occur during the first weeks of IM; this is further supported by studies in EBV-infected mice with human immune-system components.45
Another striking feature of the IM-associated NK-cell response is the persistence of elevated frequencies of the CD56dim NKG2A+ KIR- NK cells for up to 6 months after CD8+ T-cell numbers have normalized. Nevertheless, we observed no difference in the peripheral blood frequencies of these NK cells between EBV-seropositive and EBV-seronegative control individuals, contrasting the situation after CMV infection where late differentiated NK cells persist at increased levels.32 This might be explained by compartmentalized NK-cell accumulation during asymptomatic EBV infection, as has been proposed for EBV-specific T-cell responses in the tonsils,47,48 and suggests that the acute symptomatic EBV infection systemically imprints the NK-cell compartment differently. Accordingly, CD56bright NKG2A+ and mostly KIR- NK cells were found enriched in tonsils of EBV-seropositive compared with EBV-seronegative individuals.49 Indeed, this could result from ongoing lytic EBV replication at these sites in asymptomatic EBV carriers.46 Additionally, we determined that the increased CD56dim NKG2A+ KIR- NK-cell numbers during acute IM were caused by the selective proliferation of this subset. Such selectivity has not been reported to our knowledge during other acute viral infections. Because the CD56dim NKG2A+ KIR− NK subset only actively proliferated during the acute phase of IM and was only increased in absolute numbers at this stage, the persistently increased frequency of this subset might be caused by either a longer survival of these NK cells in peripheral blood or a continuous EBV-driven proliferation in tissues followed by recruitment and accumulation in the peripheral blood. Indeed, the numbers of EBV-infected B cells quickly decrease in the peripheral blood after acute IM,50 and EBV turns off antigen expression in these cells.51 Thus, an EBV-driven proliferation of NK cells during IM convalescence might not be expected in peripheral blood. Nevertheless, IM patients exhibit prolonged oral EBV shedding up to 1 year after IM,14,52 indicating that EBV replication occurs in the oropharynx. This, in turn, may result in local EBV-driven proliferation of NK cells after IM that are subsequently recruited in peripheral blood.
Several lines of evidence support the hypothesis that EBV-infected B cells, and not proinflammatory cytokines, directly drive the unique proliferation of early-differentiated NK cells: first, a similar expansion is not observed in patients with IM-like symptoms; second, the precursor CD56bright NK-cell subset, which possesses strong proliferative responses to cytokines, does not display an enhanced proliferation during acute IM; third, the frequency of proliferating early-differentiated NK cells positively correlates with EBV loads in blood cells. On the other hand, we did not observe any correlation between overall NK-cell counts and viral load. These findings are in conflict with previous studies reporting a negative correlation between NK-cell frequencies and counts and cellular EBV DNA levels41 or a positive correlation between NK-cell counts and EBV DNA levels in whole blood.14 Hence, acute IM elicits the proliferation of CD57- CD56dim NKG2A+ KIR− NK cells, which then differentiate to become CD57 positive. A fraction of this subset might represent antigen-experienced NK cells that specifically responded to EBV-infected cells in a similar fashion as during MCMV infection of experimental mice in which these NK cells are suggested to constitute memory NK cells.2,53
These early-differentiated NK cells exhibit enhanced degranulation against EBV-infected B cells with lytic reactivation. Reduced HLA-E-mediated inhibitory signals on EBV-infected cells expressing lytic antigens might lead to preferential recognition by NKG2A+ NK cells and could be caused by decreased availability of class I leader signal peptide39 or via direct modulation of HLA-E by EBV-derived peptides.54 In addition to these diminished inhibitory signals, we and others have observed an increased expression of activating ligands in lytic EBV infection,39 and NKG2D and DNAM1 have been identified as the main activating NK-cell receptors for the recognition of B cells with lytic EBV infection in vitro.39 In good agreement, loss of NKG2D function has been found to confer susceptibility to uncontrolled EBV infection and neoplasia in XMEN patients in vivo.55 How lytic EBV infection leads to the upregulation of activating ligands, however, remains unclear. NKG2D ligands have been reported to be upregulated on induction of the DNA damage response and cytosolic DNA recognition.56 Although EBV, like other herpes viruses, packages its DNA into the viral capsid in the nucleus during lytic replication, some of it might become accessible during capsid transit through the cytosol and trigger this pathway of NKG2D ligand upregulation. Newly infected B cells transiently express several lytic antigens, mostly from the immediate early and early lytic genes,57 and some of these antigens might contribute to the proliferation of NKG2A+ KIR− NK cells on EBV infection of CBMCs.
We suggest that CD56dim NKG2A+ KIR- NK cells preferentially recognize autologous B cells with lytic EBV infection, and that recognition of lytic EBV replication drives the characteristic NK-cell accumulation that we observed in acute IM patients. Because IM only manifests in older children, adolescents, and young adults with decreased frequencies of this herein newly described EBV-reactive NK-cell subset, we postulate an age-dependent impaired NK-cell-mediated immune control of EBV infection as one possible cause of IM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the pediatric patients and donors who provided clinical samples. The authors also thank Roberto Speck for the gift of CBMC samples.
This work was supported by the National Institutes of Health, National Cancer Institute (R01CA108609), the Sassella Foundation (10/02, 11/02, and 12/02), Cancer Research Switzerland (KFS-02652-08-2010), the Cancer League of the Canton of Zürich, the Association for International Cancer Research (11–0516), KFSPMS and KFSPHLD of the University of Zürich, the Vontobel Foundation, the Baugarten Foundation, the EMDO Foundation, the Sobek Foundation, Fondation Acteria, Novartis, the Walter L. and Johanna Wolf Foundation, and the Swiss National Science Foundation (310030_143979, CRSII3_136241, 310030_135028), and a fellowship from the Children's Research Center of the University Children’s Hospital Zurich (10353) (T.A.).
Authorship
Contribution: T.A. designed and performed research; A.L., A.M., V.B., and K.-J.M. performed experiments and analyzed data; C.M., O.C., and D.N. designed research; S.U., G.S., C.G., and C.B. contributed essential information or vital reagents; T.A. and O.C. analyzed data; and T.A., C.M., O.C., and D.N. wrote the manuscript.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: David Nadal, University Children’s Hospital of Zurich, Zurich, Switzerland; e-mail: david.nadal@kispi.uzh.ch; and Obinna Chijioke, Institute of Experimental Immunology, University of Zurich, Zurich, Switzerland; e-mail: chijioke@immunology.uzh.ch.
References
Author notes
O.C. and D.N. contributed equally to this study and are both senior authors.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal