Key Points
Mice lacking ERFE have more severe and prolonged AI.
ERFE suppresses hepcidin and mobilizes iron to accelerate recovery from AI.
Abstract
Erythroferrone (ERFE) is an erythropoiesis-driven regulator of iron homeostasis. ERFE mediates the suppression of the iron-regulatory hormone hepcidin to increase iron absorption and mobilization of iron from stores. We examined the role of ERFE in the recovery from anemia of inflammation (AI) induced by injection of heat-killed Brucella abortus. B abortus–treated wild-type mice developed a moderate anemia and reached nadir hemoglobin 14 days after injection and partially recovered by 28 days. We observed that Erfe expression in the bone marrow and the spleen was greatly increased during anemia and peaked at 14 days after injection, a time course similar to serum erythropoietin. To determine whether ERFE facilitates the recovery from anemia, we analyzed Erfe-deficient mice injected with B abortus. Compared with wild-type mice, Erfe-deficient mice exhibited a more severe anemia, had higher hepcidin levels and consequently lower serum iron concentration on days 14 and 21, and manifested impaired mobilization of iron from stores (liver and spleen). Erfe−/− mice eventually compensated by further stimulating erythropoiesis and reticulocyte production. Thus, ERFE contributes to the recovery from AI by suppressing hepcidin and increasing iron availability.
Introduction
Anemia of inflammation (AI) is a common feature of inflammatory disorders, including connective tissue diseases, infections, certain cancers, and chronic kidney disease.1 AI is characterized by a normocytic normochromic anemia with a shortened erythrocyte lifespan and impaired erythropoiesis, despite adequate levels of circulating erythropoietin (EPO).2 Disordered iron homeostasis is consistently observed in AI and is manifested by hypoferremia with intact iron stores1 but decreased iron availability for erythrocyte production.
The liver-produced hormone hepcidin is the main circulating regulator of iron absorption and tissue distribution.3 It acts by binding to ferroportin, the sole known cellular iron exporter, leading to ubiquitination, endocytosis, and degradation of ferroportin in lysosomes.4,5 Under the influence of high hepcidin concentrations, ferroportin is depleted from cell membranes, iron is retained in cells that export iron to plasma, and the flow of iron into plasma is decreased, which causes or contributes to iron-restrictive anemia in inflammatory disorders, chronic kidney diseases, cancer, and iron-refractory iron deficiency anemia.3 Conversely, when hepcidin production is reduced, stabilization of ferroportin at the cellular membrane promotes the absorption of dietary iron in the duodenum, increases the release of iron from macrophages that recycle old erythrocytes and other cells, and allows the mobilization of stored iron from hepatocytes.
In inflammatory disorders and during infection, hepcidin synthesis is stimulated by proinflammatory cytokines, most prominently interleukin-6 via the Janus kinase 2–signal transducer and activator of transcription 3 pathway.4,6,7 Increased hepcidin causes hypoferremia and inadequate iron supply for erythropoiesis. The mouse model of systemic inflammation induced by heat-killed Brucella abortus (BA) allows the study of recovery from inflammation-induced anemia in mice.8-11 Mice exhibit a transient but severe anemia and elevated EPO levels 14 days after injection of BA followed by the suppression of hepcidin and partial recovery from anemia by 28 days. Wild-type (WT) mice injected with BA develop a very rapid inflammatory response associated with increased hepcidin production 6 hours after BA administration and present decreased serum iron concentration and transferrin saturation, typical of iron-restricted anemia. The role of hepcidin in AI is confirmed by the milder anemia and the faster recovery of mice lacking interleukin-6 or hepcidin compared with their WT counterparts.10,11
We recently described the new erythroid factor erythroferrone (ERFE), which is responsible for early hepcidin suppression during erythropoietic activity stimulated by endogenous or exogenous EPO.12 We showed that Erfe-deficient mice fail to suppress hepcidin after erythropoietic stimulation resulting in a significant delay in the recovery from hemorrhage-induced anemia. In view of the increased concentrations of EPO observed during recovery from BA-induced anemia and the faster recovery of hepcidin-deficient mice, we examined the role of ERFE-mediated suppression of hepcidin in the recovery from AI.
Methods
Animal model of AI
Experiments were conducted in accordance with guidelines by the National Research Council and were approved by the University of California, Los Angeles. Male mice were used in this study. WT C57BL/6J males were purchased from The Jackson Laboratory (Bar Harbor, ME). Erfe+/− mice on a mixed Sv129/C57BL/6 background (Fam132btm1Lex) were generated by Lexicon Pharmaceuticals13 and were obtained from the Mutant Mouse Regional Resource Center at the University of California, Davis (strain B6;129S5-Fam132btm1Lex/Mmucd, ID MMRRC:032289-UCD). Erfe−/− mice were generated by crossing Erfe+/− mice and subsequently backcrossed onto C57BL/6 background using marker-assisted accelerated backcrossing and maintained at UCLA. Mice were maintained on a standard chow (270 ppm iron; Harlan Teklad, Indianapolis, IN) until the age of 6 weeks and were then switched to an adequate iron diet (50 ppm iron; Harlan Teklad) for 10 days prior to injection of BA or saline as previously described.10 To induce AI, animals were injected intraperitoneally with 2.5 × 108 particles per mouse of a commercial heat-killed BA preparation (strain 1119-3; US Department of Agriculture, Animal and Plant Health Inspection Service, National Veterinary Services Laboratories). Control mice were injected intraperitoneally with normal saline. During the experiments, mice were maintained on a 50 ppm diet and studied on days 0, 7, 13, 21, and 28 after injection (6-8 mice per time point in each group). For the kinetics of hepcidin response experiment, 6 WT and 6 Erfe−/− mice were injected with BA and 4 WT and 4 Erfe−/− mice were injected with saline. Mice were then monitored during 28 days, and blood was sampled every week by retro-orbital puncture (50-100 μL).
Measurement of iron and hematologic parameters
Serum iron, spleen, and liver nonheme iron concentrations were determined as previously described,14 using acid treatment followed by a colorimetric assay for iron quantitation (Sekisui Diagnostics, Charlottetown, Canada). Complete blood counts were obtained with a HemaVet blood analyzer (Drew Scientific). Reticulocytes were counted by flow cytometry. Blood (5 μL) was added to 1 mL of thiazole orange in phosphate-buffered saline (PBS) with 0.1% sodium azide (BD Retic-Count; BD Bioscience, San Jose, CA) and incubated at room temperature for 1 hour. The percentage of red fluorescent reticulocytes (Retic %) was measured by flow cytometry according to the manufacturer’s instructions. Absolute reticulocyte count was calculated by multiplying the Retic % by red blood cell (RBC) count. Reticulocyte size was determined using forward scatter.
Quantitation of mRNA levels
Total RNA from mouse tissues was extracted using TRIzol (Invitrogen). Complementary DNA was synthesized using iScript (Bio-Rad). Quantitative polymerase chain reactions were prepared with Sso advanced SYBR Green supermix (Bio-Rad) (primers indicated in supplemental Table 1, available on the Blood Web site) and run in duplicate on a CFXconnect Instrument (Bio-Rad). Hamp, Erfe, Saa1, and Gypa messenger RNA (mRNA) transcript abundance was normalized to the reference gene Rpl4. Rpl4 was chosen as the reference gene using Genevestigator.15 Results are expressed as −ΔCt ± standard error of the mean (SEM) (ie, the cycle threshold differences between reference and target genes within each group of mice). Expression ratio and SEM values of Hamp transcripts in Erfe-deficient mice relative to controls and normalized to the reference gene Rpl4 mRNA were calculated using REST.16 Statistical significance was determined using randomization tests.
Enzyme-linked immunosorbent assays
Mouse hepcidin-1 monoclonal antibodies Ab2B10 (capture), AB2H4-HRP (detection), and synthetic mouse hepcidin-25 were a generous gift from Amgen (Thousand Oaks, CA).17 High-binding 96-well enzyme immunoassay plates (Corning) were coated overnight at room temperature with 50 μL/well of 3.6 μg/mL Ab2B10 in 0.2 M carbonate-bicarbonate buffer (pH 9.4) (Pierce). Plates were washed twice with wash buffer (PBS, 0.5% Tween-20) and blocked for 45 minutes with 200 μL/well blocking buffer (PBS, 1% bovine serum albumin, 1% normal goat serum, 0.5% Tween-20). Samples and standards were added and incubated 1 hour at room temperature. After 4 washes, plates were incubated for 1 hour with 50 μL/well of 130 ng/mL Ab2H4-HRP, washed 4 times, and then developed with 100 μL/well Ultra-TMB substrate (Thermo Scientific) for 30 minutes in the dark at room temperature. The reaction was stopped by adding 50 μL 2 M sulfuric acid, and the absorbance was measured at 450 nm. Serum EPO was measured using a mouse EPO quantikine set (R&D Systems), according to the manufacturer’s instructions.
Statistical analysis
The statistical significance of differences between groups was evaluated using Sigmaplot 11.0 package (Systat Software, San Jose, CA). The Student t test was used to compare 2 groups of normally distributed data. The Mann-Whitney rank-sum test was used to compare data that were not normally distributed. P < .05 in a 2-tailed test was considered as statistically significant.
Results
ERFE expression is stimulated during BA-induced AI
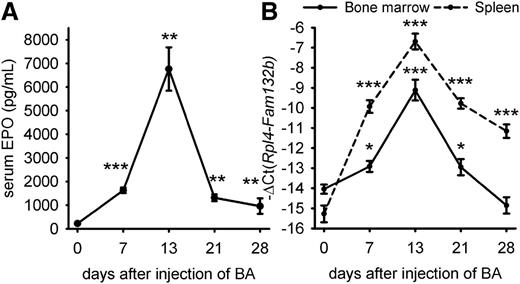
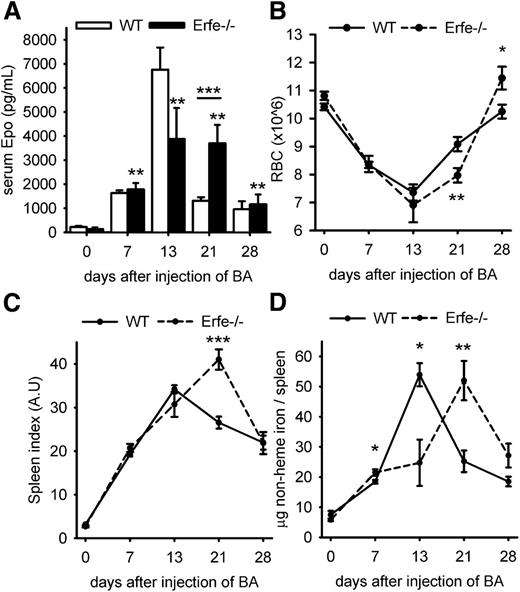
We first examined the time course of EPO over 28 days after injection of WT mice with 2.5 × 108 particles of heat-killed BA (Figure 1A). EPO levels were elevated by day 7 and peaked on day 13 (median concentration = 6039 pg/mL vs 168 pg/mL at day 0). EPO levels then gradually decreased but were still higher than baseline on days 21 and 28 (median concentration = 1303 and 680 pg/mL, respectively, vs 168 pg/mL at baseline). Because the expression of ERFE-encoding Fam132b mRNA is stimulated by EPO, we assessed ERFE transcript abundance in the bone marrow and spleen after injection of BA (Figure 1B). We found that Erfe (Fam132b) mRNA expression mirrored serum EPO concentrations and was maximally induced 32-fold in the bone marrow and >300-fold in the spleen 21 days after injection of BA compared with control mice at day 0. By day 28, Erfe mRNA levels returned to normal in the bone marrow, but not in the spleen. Erfe is expressed by differentiating erythroblasts,12 and high levels of splenic Erfe after BA injection may result from expanded extramedullary erythropoiesis.10,11 To determine the relative contributions of changes in erythroid precursor numbers and changes in Erfe (Fam132b) mRNA per erythroid precursor, we analyzed expression of the erythroid marker glycophorin A (Gypa), as well as Erfe expression relative to Gypa (supplemental Figure 1). By day 7, Gypa mRNA expression was increased in the spleen, confirming expansion of extramedullary erythropoiesis (supplemental Figure 1A). The rise of Erfe mRNA expression in the spleen by day 7, even when normalized to Gypa (supplemental Figure 1B), demonstrated that Erfe increase resulted from not only increased numbers of splenic erythroid precursors but also increased Erfe mRNA per erythroid precursor. The subsequent changes in Erfe mRNA reflect predominantly the expression of Erfe per erythroid precursor, as the spleen Gypa expression remained stable between days 7 and 28. In the marrow, the initial rise in Erfe mRNA occurs despite the suppression of erythroid precursors by day 7, as reflected by decreased Gypa expression. Similarly to the spleen, the subsequent changes in marrow Erfe expression are substantially driven by the changes in production of Erfe mRNA per erythroid precursor.
Time-course of serum EPO concentration and ERFE (Fam132b) mRNA expression after injection of heat-killed BA. Serum EPO levels (A) and ERFE mRNA expression in the bone marrow and spleen (B) were elevated by 7 days. Both serum EPO and Erfe mRNA reached a peak at 13 days and remained above baseline for 21 to 28 days. Data shown are means ± SEM and were compared for each time point to values for control mice at t = 0 (n = 6-7 per time point) by 2-tailed Student t test (***P < .001, **P < .01, *P < .05).
Time-course of serum EPO concentration and ERFE (Fam132b) mRNA expression after injection of heat-killed BA. Serum EPO levels (A) and ERFE mRNA expression in the bone marrow and spleen (B) were elevated by 7 days. Both serum EPO and Erfe mRNA reached a peak at 13 days and remained above baseline for 21 to 28 days. Data shown are means ± SEM and were compared for each time point to values for control mice at t = 0 (n = 6-7 per time point) by 2-tailed Student t test (***P < .001, **P < .01, *P < .05).
Erfe-deficient mice develop more severe anemia than WT mice during recovery after BA injection
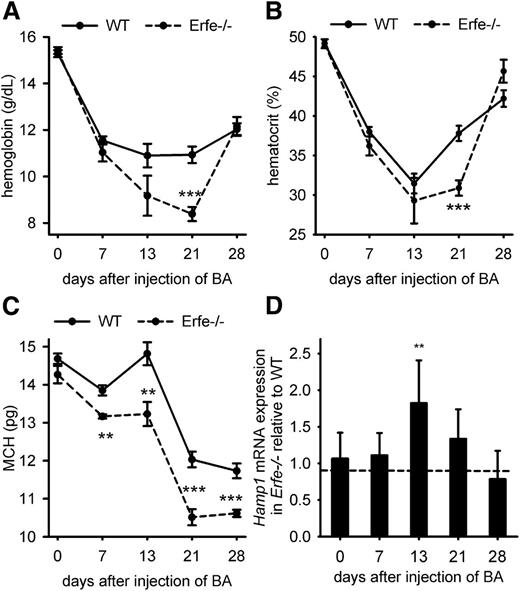
To determine what role ERFE plays in AI, we compared the recovery from BA-induced anemia in WT mice and mice lacking ERFE. On day 21 after the inflammatory stimulus, Erfe-deficient mice showed significantly lower hemoglobin concentration (8.3 vs 11 g/dL; Figure 2A) and lower hematocrit (31 vs 38%, Figure 2B) than WT mice. Mean corpuscular hemoglobin (MCH; Figure 2C), MCH concentration, and mean corpuscular volume (supplemental Figure 2) were also consistently reduced throughout the time course in Erfe−/− mice compared with WT mice, suggesting more severe iron restriction. Thus, mice deficient for ERFE exhibit a more pronounced anemia and a delayed recovery from BA-induced anemia relative to WT mice. However, both groups initiate a return toward normal by day 28, suggesting that Erfe-deficient mice eventually compensate for the absence of ERFE.
Erfe-deficient mice exhibited a more severe AI than WT mice. Hemoglobin (A), hematocrit (B), and MCH (C) were compared between WT and Erfe-deficient mice at t = 0, 7, 13, 21, and 28 days after injection of BA. Erfe−/− mice had lower hemoglobin concentration and lower hematocrit on day 21 than WT mice. MCH was also lower at days 7, 13, 21, and 28 in Erfe−/− mice compared with WT mice. Erfe-deficient mice had higher hepcidin levels 13 days after injection of BA compared with WT mice (D) (see also supplemental Figure 3A). Expression ratios ± SEM of Rpl4-normalized Hamp transcripts in Erfe−/− mice relative to WT controls were calculated using REST. Statistical significance was determined using randomization. Data shown for hemoglobin, hematocrit, and MCH are means ± SEM and were compared between WT and Erfe−/− mice (n = 6-7 per time point) by 2-tailed Student t test at each time point (***P < .001, **P < .01, *P < .05).
Erfe-deficient mice exhibited a more severe AI than WT mice. Hemoglobin (A), hematocrit (B), and MCH (C) were compared between WT and Erfe-deficient mice at t = 0, 7, 13, 21, and 28 days after injection of BA. Erfe−/− mice had lower hemoglobin concentration and lower hematocrit on day 21 than WT mice. MCH was also lower at days 7, 13, 21, and 28 in Erfe−/− mice compared with WT mice. Erfe-deficient mice had higher hepcidin levels 13 days after injection of BA compared with WT mice (D) (see also supplemental Figure 3A). Expression ratios ± SEM of Rpl4-normalized Hamp transcripts in Erfe−/− mice relative to WT controls were calculated using REST. Statistical significance was determined using randomization. Data shown for hemoglobin, hematocrit, and MCH are means ± SEM and were compared between WT and Erfe−/− mice (n = 6-7 per time point) by 2-tailed Student t test at each time point (***P < .001, **P < .01, *P < .05).
Erfe-deficient mice have impaired hepcidin regulation during AI
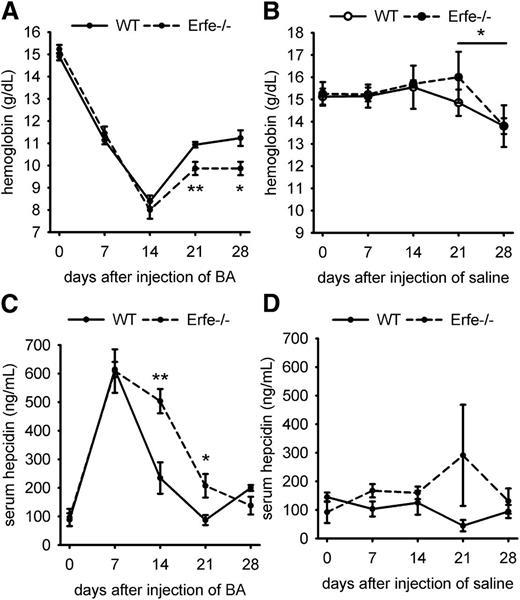
The synthesis of the hepatic hormone hepcidin is stimulated by inflammation, but hepcidin is suppressed by ERFE during increased erythropoietic activity, which facilitates iron availability for new erythrocyte production.12 Ablation of hepcidin during BA-induced AI in mice relieves iron restriction and improves the anemia.10 However, Erfe-deficient mice fail to suppress hepcidin after phlebotomy or EPO injection.12 The lack of hepcidin suppression in Erfe-deficient mice after BA injection could therefore be responsible for the delayed recovery compared with WT animals. We therefore measured hepatic hepcidin mRNA levels after BA injection. On day 13, despite worse anemia in Erfe−/− mice and lesser inflammatory stimulation as shown by the expression of the inflammatory marker Saa1 (supplemental Figures 3B and 4), hepcidin mRNA expression was 2-fold higher in Erfe-deficient mice than in WT mice (Figure 2D and supplemental Figure 3). In Figure 2, each time point comprised a separate group of animals. Because baseline hepcidin levels are affected by minor changes in iron status and different cohorts of mice may vary somewhat in hepcidin levels and inflammation, we serially monitored the same WT and Erfe-deficient animals during 28 days after injection of BA (6 mice per genotype) or saline (4 mice per genotype). A total of 50 to 100 μL of blood was withdrawn by retro-orbital puncture at days 0, 7, 14, 21, and 28, and complete blood count and serum hepcidin measurements were performed at each time point. We confirmed that Erfe-deficient mice had significantly lower hemoglobin concentration than WT mice on days 21 and 28 after injection of BA (Figure 3A). For both WT and Erfe-deficient mice, repeated phlebotomies may have delayed the recovery between days 21 and 28. Indeed, even after saline injection, both WT and Erfe-deficient mice exhibited slightly lower hemoglobin concentration at day 28 compared with day 21 (Figure 3B). We observed that Erfe-deficient mice decreased their hepcidin levels much less than WT mice 14 days after BA injection (Figure 3C; mean concentration was 503 ng/mL in Erfe−/− mice vs 234 ng/mL in WT mice). As inflammation resolved (supplemental Figure 4), hepcidin levels were reduced in Erfe−/− mice between days 14 and 21 after injection but remained significantly higher than those in WT mice (Figure 3C; mean concentration was 207 ng/mL in Erfe−/− mice vs 87 ng/mL in WT mice). The weekly analyses did not significantly influence hepcidin levels in saline-treated mice (Figure 3D). These results demonstrate that ERFE is necessary for adaptive hepcidin regulation during recovery from AI.
Time course of hemoglobin and serum hepcidin concentrations after saline or BA injection in WT and Erfe-deficient mice.Erfe−/− mice had lower hemoglobin concentration than WT mice at 21 and 28 days after injection of BA (A). Repetitive phlebotomies caused a slight decrease in hemoglobin by 28 days in both WT and Erfe−/− mice treated with saline (B). Serum hepcidin concentration was higher on days 14 and 21 after BA injection in Erfe−/− mice compared with WT mice (C) but was unchanged in saline-injected mice (D). Data shown are means ± SEM and were compared for each time point between WT and Erfe−/− mice (n = 6 per time point) by 2-tailed Student t test (**P < .01, *P < .05).
Time course of hemoglobin and serum hepcidin concentrations after saline or BA injection in WT and Erfe-deficient mice.Erfe−/− mice had lower hemoglobin concentration than WT mice at 21 and 28 days after injection of BA (A). Repetitive phlebotomies caused a slight decrease in hemoglobin by 28 days in both WT and Erfe−/− mice treated with saline (B). Serum hepcidin concentration was higher on days 14 and 21 after BA injection in Erfe−/− mice compared with WT mice (C) but was unchanged in saline-injected mice (D). Data shown are means ± SEM and were compared for each time point between WT and Erfe−/− mice (n = 6 per time point) by 2-tailed Student t test (**P < .01, *P < .05).
Erfe-deficient mice exhibit more severe iron restriction during AI
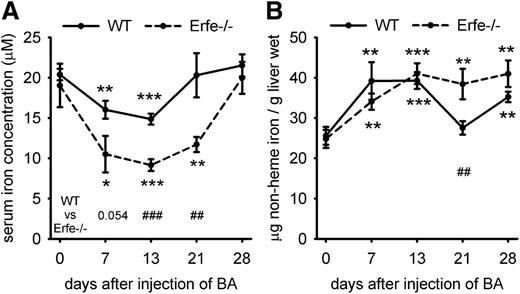
Because Erfe ablation interfered with appropriate regulation of hepcidin during BA-induced anemia, we evaluated iron concentrations in serum and storage organs. Serum iron concentrations decreased in WT and Erfe-deficient mice between days 0 and 13 after injection of BA (Figure 4A), but the lack of appropriate hepcidin suppression in Erfe-deficient mice led to a much more severe decrease in serum iron concentration compared with WT mice on days 13 and 21. Increased hepcidin expression in both WT and Erfe-deficient mice prevented iron mobilization from stores as shown by increased liver iron content at 7 and 13 days after inflammatory stimulus (Figure 4B). In WT mice, liver iron levels returned to normal by day 21 as hepcidin expression decreased, but Erfe-deficient mice had higher liver iron content than WT mice at day 21, confirming a prolonged impairment of iron mobilization from stores. In WT mice, reduced hepcidin levels by day 21 caused iron hyperabsorption leading to increased serum iron and liver iron concentration by day 28. Taken together, these results confirm that hepcidin dysregulation in Erfe-deficient mice leads to impaired mobilization of iron from the stores and a significant delay in recovery from AI.
Serum and liver iron concentrations in WT and Erfe-deficient mice after injection of BA. Serum iron concentration was decreased between days 0 and 13 after injection of BA in WT and Erfe−/− mice and was still reduced on day 21 in Erfe−/− mice. Compared with WT mice, Erfe-deficient mice exhibited significantly lower serum iron concentrations on days 13 and 21 (A). Liver iron content (B) was increased during recovery from anemia in WT and Erfe-deficient mice except for WT mice at day 21, where iron levels are similar to day 0. Compared with WT mice, liver iron content was significantly higher in Erfe−/− mice 21 days after infection. Data shown for serum iron concentration (A) and liver iron content (B) are means ± SEM and were compared for each time point to values for control mice at t = 0 (n = 6-7 per time point) by 2-tailed Student t test (***P < .001, **P < .01, *P < .05). Values were also compared at each time point between WT and Erfe−/− mice by 2-tailed Student t test (###P < .001, ##P < .01).
Serum and liver iron concentrations in WT and Erfe-deficient mice after injection of BA. Serum iron concentration was decreased between days 0 and 13 after injection of BA in WT and Erfe−/− mice and was still reduced on day 21 in Erfe−/− mice. Compared with WT mice, Erfe-deficient mice exhibited significantly lower serum iron concentrations on days 13 and 21 (A). Liver iron content (B) was increased during recovery from anemia in WT and Erfe-deficient mice except for WT mice at day 21, where iron levels are similar to day 0. Compared with WT mice, liver iron content was significantly higher in Erfe−/− mice 21 days after infection. Data shown for serum iron concentration (A) and liver iron content (B) are means ± SEM and were compared for each time point to values for control mice at t = 0 (n = 6-7 per time point) by 2-tailed Student t test (***P < .001, **P < .01, *P < .05). Values were also compared at each time point between WT and Erfe−/− mice by 2-tailed Student t test (###P < .001, ##P < .01).
The lack of ERFE is compensated by prolonged stimulation of erythropoiesis
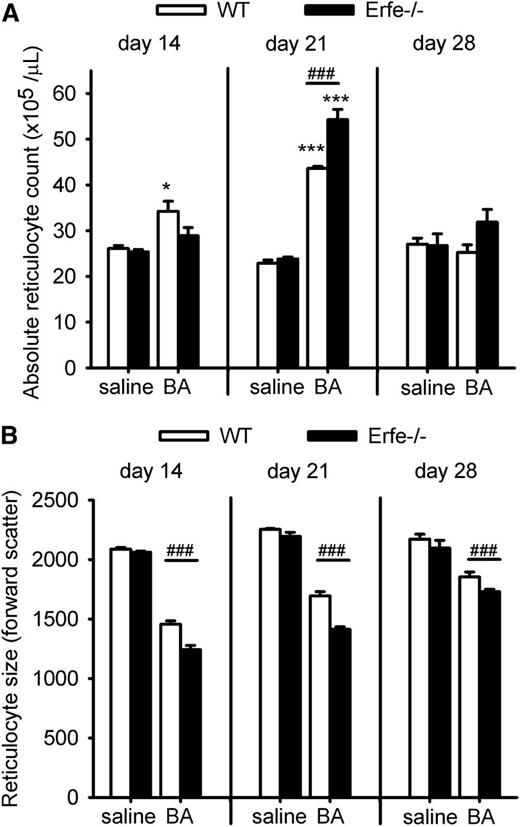
By day 28 after injection of BA, Erfe-deficient mice have anemia of similar severity as WT mice (Figure 2A). We next investigated the mechanism by which Erfe-deficient mice compensate for the limitation of iron supply for erythropoiesis. We observed that on day 21, serum EPO concentrations remained much higher in Erfe−/− mice than in WT mice (Figure 5A; serum EPO concentration was 3111 pg/mL in Erfe−/− mice vs 1303 pg/mL in WT mice at day 21), consistent with the more severe anemia in Erfe−/− mice (Figures 2A-C and 5B). As a result of prolonged stimulation of erythropoiesis and increased RBC production, Erfe-deficient mice reached a higher RBC count than WT mice by day 28 (Figure 5B; RBC count was 11.4 × 106 in Erfe−/− mice vs 10.2 × 106 in WT mice). Spleen index (the ratio between the weight of the spleen in milligram relative to the total body weight in grams) revealed that the spleen size was increased throughout the time course for both genotypes, but the spleen was significantly larger in Erfe-deficient mice compared with WT at day 21 (Figure 5C). This suggests more active erythropoiesis in the spleen of Erfe−/− mice compared with WT mice. The spleen iron content gradually increased between day 0 and day 13 as new red cells were produced through extramedullary erythropoiesis in the spleen. The spleen iron content then progressively decreased until 28 days, probably because of the depletion of iron stores for hemoglobin synthesis (Figure 5D). Interestingly, the spleen iron content in Erfe-deficient mice mirrored the course described in WT mice, but with a 1-week delay. To compare the rate of erythropoiesis between WT and mutant mice, we then examined the reticulocyte production 14, 21, and 28 days after BA injection. At 14 days after inflammatory stimulus, WT mice were producing more reticulocytes than saline mice (Figure 6A), but no difference was observed in Erfe-deficient mice. The formation of new reticulocytes was greatly increased on day 21 for both genotypes (P < .001 compared with saline mice) but was significantly higher in Erfe−/− mice compared with WT mice. RBC counts and Retic %, which were used to calculate absolute reticulocyte count for both saline and BA-treated mice, are shown in supplemental Figure 5. As expected, given the inflammation-induced iron-restriction, newly synthesized reticulocytes were smaller than normal throughout the time course in WT and Erfe-deficient mice (Figure 6B). However, Erfe-deficient mice produced even smaller cells than WT (Figure 6B) and the red cell distribution width was slightly higher 28 days after the inflammatory stimulus (supplemental Figure 6), confirming that erythropoiesis in these mice was more iron-restricted than in their WT counterparts. Thus Erfe-deficient mice compensate for hepcidin dysregulation and more restricted iron supply by increased EPO and prolonged stimulation of erythropoiesis, resulting in delayed but effective partial recovery from anemia.
Erfe-deficient mice compensate for hepcidin dysregulation by prolonged erythropoietic stimulation. (A) High EPO concentrations were sustained at 21 days in Erfe-deficient mice (black bars, mean EPO concentration = 3111 pg/mL) compared with WT mice (white bars, mean EPO concentration = 1303 pg/mL, P = .001, shown previously in Figure 1A). Erfe−/− mice had a higher RBC count (B) and spleen index (C) on day 28 than WT mice (spleen index = spleen weight in mg/body weight in g). (D) Nonheme spleen iron content peaked earlier in WT than in Erfe−/− mice. (A) Serum EPO levels are shown as means ± SEM and were compared for each time point to values for control mice at t = 0 (n = 6-7 per time point) by 2-tailed Student t test. Data shown for RBC (B), spleen index (C), and total spleen iron (D) are means ± SEM and were compared between WT and Erfe−/− mice for each time point (n = 6-7 per time point) by 2-tailed Student t test (***P < .001, **P < .01, *P < .05). WT data shown in panel A are identical to those from Figure 1A.
Erfe-deficient mice compensate for hepcidin dysregulation by prolonged erythropoietic stimulation. (A) High EPO concentrations were sustained at 21 days in Erfe-deficient mice (black bars, mean EPO concentration = 3111 pg/mL) compared with WT mice (white bars, mean EPO concentration = 1303 pg/mL, P = .001, shown previously in Figure 1A). Erfe−/− mice had a higher RBC count (B) and spleen index (C) on day 28 than WT mice (spleen index = spleen weight in mg/body weight in g). (D) Nonheme spleen iron content peaked earlier in WT than in Erfe−/− mice. (A) Serum EPO levels are shown as means ± SEM and were compared for each time point to values for control mice at t = 0 (n = 6-7 per time point) by 2-tailed Student t test. Data shown for RBC (B), spleen index (C), and total spleen iron (D) are means ± SEM and were compared between WT and Erfe−/− mice for each time point (n = 6-7 per time point) by 2-tailed Student t test (***P < .001, **P < .01, *P < .05). WT data shown in panel A are identical to those from Figure 1A.
During recovery from BA-induced anemia, Erfe-deficient mice show delayed and more iron-restricted reticulocytosis than WT mice. Absolute reticulocyte count (A) was significantly increased on day 14 only in WT mice, but by day 21, Erfe-deficient mice had higher reticulocyte count than WT mice. Using the forward scatter as an assessment of the reticulocyte size (B), we found that both WT and Erfe−/− mice injected with BA were producing smaller cells than their respective saline controls. Erfe−/− mice produced even smaller reticulocytes than WT at days 14, 21, and 28 (B). Data shown are means ± SEM and were compared for each time point within the same genotype between saline and BA-treated mice by 2-tailed Student t test (*P < .05, ***P < .001). Values were also compared at each time point between WT and Erfe-deficient mice (n = 6 per time point) by 2-tailed Student t test (###P < .001).
During recovery from BA-induced anemia, Erfe-deficient mice show delayed and more iron-restricted reticulocytosis than WT mice. Absolute reticulocyte count (A) was significantly increased on day 14 only in WT mice, but by day 21, Erfe-deficient mice had higher reticulocyte count than WT mice. Using the forward scatter as an assessment of the reticulocyte size (B), we found that both WT and Erfe−/− mice injected with BA were producing smaller cells than their respective saline controls. Erfe−/− mice produced even smaller reticulocytes than WT at days 14, 21, and 28 (B). Data shown are means ± SEM and were compared for each time point within the same genotype between saline and BA-treated mice by 2-tailed Student t test (*P < .05, ***P < .001). Values were also compared at each time point between WT and Erfe-deficient mice (n = 6 per time point) by 2-tailed Student t test (###P < .001).
Discussion
Treatment of the underlying disease is the therapeutic approach of choice for anemia of chronic disease.1,18 However, when this is unfeasible, persistent anemia is associated with symptoms and a poorer prognosis in many conditions. A better understanding of the pathophysiology of anemia of chronic disease should facilitate new therapeutic strategies.
We recently described the identification of the erythroid mediator ERFE,12 which links erythropoiesis to iron homeostasis. ERFE, a strong suppressor of hepcidin expression, is produced in response to EPO by erythroblasts in the bone marrow and spleen. Importantly, Erfe-deficient mice failed to suppress hepcidin after hemorrhage or EPO administration, leading to a delayed recovery from hemorrhage-induced anemia.
We therefore asked whether ERFE contributed to hepcidin suppression and the recovery from AI. Using a recently described mouse model,10,11 we aimed to achieve a prolonged anemia with hemoglobin levels between 9 and 11 g/dL, similar to the common form of anemia of chronic disease. Accordingly, mice were given 2.5 × 108 particles of heat-killed BA, half the dose used in the previously described more severe models.10,11 Erfe mRNA expression was highly induced in the bone marrow and spleen of BA-treated mice, with a peak at 2 weeks after inflammatory stimulus, reflecting serum EPO concentration (Figure 1). In the bone marrow, ERFE production returned to baseline level in parallel to EPO but remained somewhat increased in the spleen, the site of active extramedullary erythropoiesis (Figure 1B). Interestingly, Erfe-deficient mice exhibited a more severe anemia than WT mice 14 to 21 days after injection of BA, suggesting that ERFE plays an important regulatory role in resolving AI (Figure 2).
In the absence of ERFE, mice showed impaired hepcidin suppression during recovery (Figures 2D and 3B) leading to a more severe and prolonged hypoferremia and impaired mobilization of iron from stores (Figure 4). However, by day 28 after the inflammatory stimulus, Erfe-deficient compensated and partially recovered similarly to WT mice (Figures 2 and 3). Apparently, the absence of ERFE was rescued by prolonged stimulation of erythropoiesis in Erfe-deficient mice as shown by elevated serum EPO concentration, spleen index, and reticulocytosis at day 21 compared with WT mice (Figures 5 and 6). However, the more pronounced hypoferremia in Erfe-deficient mice resulted in smaller reticulocytes than in WT mice, a sign of more severe iron restriction (Figure 6). In the aggregate, these results document that by suppressing hepcidin and thereby releasing iron for compensatory erythropoiesis, ERFE moderates the severity of AI and facilitates recovery. Further experiments including measurements of circulating levels of ERFE in patients with AI or administration of ERFE in BA-treated mice will be necessary to determine its exact contribution in the pathophysiology of common anemias.
The pathogenic role of increased hepcidin in iron-restricted anemias makes it a promising target for novel therapeutic approaches.19 Multiple agents directed at lowering hepcidin production or interfering with hepcidin peptide activity are under development, and several clinical trials are already underway. Given its hepcidin-suppressive activity and involvement in recovery from anemia, ERFE or related agonists are natural candidates as agents that could ameliorate hepcidin-induced iron restriction in inflammatory disorders, chronic kidney disease, or cancer.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Victoria Gabayan for her invaluable work with the mouse breeding. Flow cytometry was performed in the University of California at Los Angeles (UCLA) Jonsson Comprehensive Cancer Center (JCCC) and the Center for AIDS Research Flow Cytometry Core Facility that is supported by National Institutes of Health National Cancer Institute (CA-16042), National Institute of Allergy and Infectious Diseases (AI-28697), and by the JCCC, the UCLA AIDS Institute, and the David Geffen School of Medicine at UCLA.
This research was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK 065029 (T.G.) and the ASH scholar award (L.K.).
Authorship
Contribution: L.K. designed and performed the experiments, analyzed the data, and wrote the paper; G.J. assisted with experiments; and E.N. and T.G. supervised the project and wrote the paper.
Conflict-of-interest disclosure: E.N. and T.G. are shareholders and scientific advisors of Intrinsic LifeSciences and Merganser Biotech. T.G. is a consultant to Keryx Biopharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Tomas Ganz, Department of Medicine, UCLA, 10833 LeConte Ave, CHS 37-131, Los Angeles, CA 90095; e-mail: tganz@mednet.ucla.edu; and Léon Kautz, Department of Medicine, UCLA, 10833 LeConte Ave, CHS 37-131, Los Angeles, CA 90095; e-mail: lkautz@mednet.ucla.edu.