In this issue of Blood, van Eijk et al investigate a synthetic compound that inhibits the iron-regulatory hormone hepcidin to fight anemia of inflammation in human endotoxemia.1
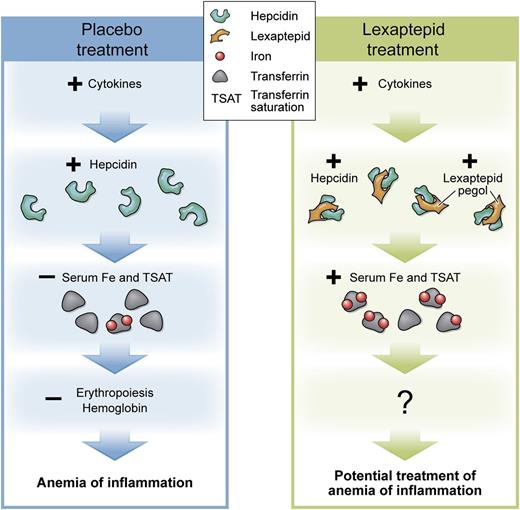
Effects of placebo or lexaptepid treatment on inflammatory-mediated hepcidin induction and systemic iron homeostasis. With placebo, acute or chronic inflammations as well as neoplastic disease cause an induction of cytokines. Cytokines, in turn, induce the expression of the iron-regulatory hormone hepcidin. Hepcidin degrades the iron-export channel ferroportin, so that hypoferremia develops. Erythropoiesis and hemoglobin levels decrease. Anemia of inflammation ensues. Patients treated with lexaptepid show the same induction of cytokines. Hepcidin is induced but bound by lexaptepid. The hepcidin-lexaptepid complex is biologically inactive and prevents hypoferremia. Therefore, lexaptepid might be a possible treatment of anemia of inflammation. The effects on erythropoiesis and hemoglobin levels have yet to be determined in clinical trials. TSAT, transferrin saturation. Professional illustration by XavierStudio.
Effects of placebo or lexaptepid treatment on inflammatory-mediated hepcidin induction and systemic iron homeostasis. With placebo, acute or chronic inflammations as well as neoplastic disease cause an induction of cytokines. Cytokines, in turn, induce the expression of the iron-regulatory hormone hepcidin. Hepcidin degrades the iron-export channel ferroportin, so that hypoferremia develops. Erythropoiesis and hemoglobin levels decrease. Anemia of inflammation ensues. Patients treated with lexaptepid show the same induction of cytokines. Hepcidin is induced but bound by lexaptepid. The hepcidin-lexaptepid complex is biologically inactive and prevents hypoferremia. Therefore, lexaptepid might be a possible treatment of anemia of inflammation. The effects on erythropoiesis and hemoglobin levels have yet to be determined in clinical trials. TSAT, transferrin saturation. Professional illustration by XavierStudio.
Anemia is one of the major public health burdens. The World Health Organization estimates the prevalence of anemia at 2 billion people worldwide. Prevalence of anemia in 2010 was estimated at 32.9% in a systemic analysis of the global anemia burden from 1990 to 2010. Anemia caused 68.4 million years lived with disability.2 Iron deficiency anemia is the most frequent form of anemia, typically due to nutritional deficiency and characterized by intact regulation of iron homeostasis and decreased serum iron levels and tissue iron stores. Anemia of inflammation, also known as the anemia of chronic disease, is the second most common form of anemia. In anemia of inflammation, acute or chronic infections or neoplastic disease cause an immune-modulatory response in patients with an induction of cytokine expression. Cytokines, in turn, lead to an increase of the iron-regulatory hormone hepcidin. Synthesized mainly by the liver, hepcidin maintains systemic iron homeostasis by posttranslationally downregulating levels of the sole iron exporter, ferroportin. Hepcidin binds to ferroportin and causes internalization and degradation of the iron exporter. As the 3 sites of ferroportin expression are duodenal enterocytes, hepatocytes, and reticuloendothelial macrophages, dietary iron absorption and iron release from tissue stores is inhibited. Anemia of inflammation ultimately ensues.3,4
Anemia of inflammation is frequent in hospitalized patients. A secondary retrospective analysis of a prospective study of 39 309 patients from 28 European nations on preoperative anemia revealed that 26.5% of women and 31.1% of men were anemic.5 If these patients undergo surgery, preoperative anemia is a risk factor associated with higher in-hospital mortality, longer stay in the hospital, and higher frequency of postoperative admission to the intensive care unit compared with patients without preoperative anemia.5
Fighting anemia therefore is of high clinical relevance and critical, independent of age and country of residence. To date, therapeutic options for anemia of inflammation include treatment of the underlying disease, blood transfusions, intravenous iron supplementation, or erythropoietin, if applicable.
Because hepcidin is induced in anemia of inflammation, researchers have tried for several years to identify a means to target and inhibit hepcidin. If hepcidin expression can be inhibited or biologically inactivated, the iron regulation of the organism remains intact. Obviously, the side effects of these treatments have yet to be investigated. Current inhibitors of hepcidin, as reviewed by Nemeth and Ganz,3 include targets of pathways of hepcidin induction (such as the small-molecule inhibitor LDN-193189),6 antihepcidin antibodies (such as the fully human antihepcidin antibody 12B9m),7 or synthetically generated hepcidin binders such as anticalins or RNA-based Spiegelmers.8
Lexaptepid pegol (lexaptepid) is a synthetically generated PEGylated l-stereoisomeric RNA aptamer. It belongs to the Spiegelmers and consists of a synthetic l-oligoribonucleotide with a 3-dimensional structure. Due to the unnatural mirror-image structure, it is of higher biological stability compared with natural d-aptamers. Lexaptepid binds hepcidin conceptually similar to antibodies, forms a complex, and thereby inhibits hepcidin.
In this issue of Blood, van Eijk and colleagues present their study on the use of the Spiegelmer lexaptepid in a first human trial.1 The efficiency of lexaptepid for inhibition of hepcidin expression was initially tested in cell culture and animal studies.9 In the current article, experimental endotoxemia was induced in 24 healthy male volunteers by intravenous Escherichia coli lipopolysaccharide (LPS) injection followed by either a single injection of lexaptepid or placebo (see figure).1 The increase in serum hepcidin levels after LPS injection was comparable in both groups. This indicates that hepcidin measurement cannot differentiate between free hepcidin and the complex of hepcidin-lexaptepid. Both groups presented with similar flu-like symptoms, increase in body temperature and laboratory markers indicative of inflammation such as C-reactive protein, leukocyte counts, and cytokine concentrations. In contrast, the decrease in serum iron levels within 9 hours after injection observed in the placebo group did not occur in patients injected with lexaptepid. These data reveal that lexaptepid does not interfere with the inflammatory reaction but disables hepcidin's ability to induce hypoferremia.
To summarize, lexaptepid is effective in preventing the first step following hepcidin induction due to endotoxemia: effective prevention of a decrease in serum iron. It is not yet known if lexaptepid prevents the development of anemia of inflammation or treat the anemia of inflammation after it is already established. The future direction is clear: Additional novel data on clinical trials with lexaptepid in patients are expected to be released soon. The authors have already presented data from a phase 2 pilot study in cancer patients (multiple myeloma, low-grade lymphoma, and chronic lymphocytic leukemia) with anemia of chronic disease at the American Association for Cancer Research.10 In this study, treatment with lexaptepid for 4 weeks resulted in a hemoglobin increase of ≥1 g/dL in 5 of 12 patients. Patients responding to the treatment also presented with an increase in reticulocyte hemoglobin.
Lexaptepid seems to be a promising drug to battle anemia of inflammation.
Conflict-of-interest disclosure: The author declares no competing financial interests.