To the editor:
Mobilized hematopoietic stem and progenitor cells (HSPCs) collected from peripheral blood (PB) is the most common source of HSPCs for stem cell transplantation, and granulocyte colony-stimulating factor (G-CSF) is the most common agent used for stem cell mobilization. Unfortunately, 5% to 30% of patients fail to mobilize sufficient stem cells for autologous stem cell transplantation in response to G-CSF.1,2 Although other modalities have been used to enhance mobilization, including adding plerixafor to G-CSF or chemomobilization, inadequate HSPC collection remains a major problem. Therefore, new strategies are needed to optimize stem cell mobilization. HSPCs express cell surface molecules such as CXCR4 and very late antigen 4 (VLA-4) that mediate their adherence to the bone marrow microenvironment via interaction with stromal derived factor 1 and vascular cell adhesion molecule (VCAM-1), respectively.1
Bortezomib (Velcade, PS-341) was the first proteasome inhibitor approved by Food and Drug Administration for the treatment of multiple myeloma. Bortezomib blocks the activation of nuclear factor-κB by preventing proteasomal degradation of IκBα.3-5 VCAM-1 promoter has 2 binding sites for nuclear factor-κB6 , and proteasome inhibitors inhibit transcription and expression of VCAM-1.4,7 In light of the importance of the VLA-4/VCAM-1 interaction in HPSC homing, we hypothesized that bortezomib could directly mobilize HPSCs. Here we show, for the first time, that bortezomib is a potent mobilizing agent in mice. Furthermore, to investigate the mechanism of mobilization, we tested the effect of bortezomib in VLA-4 knockout (VLA-4KO) and splenectomized mice. Finally, to test the role of bortezomib in combination with other approved Food and Drug Administration mobilizing agents, we tested the effect of bortezomib in combination with G-CSF and plerixafor (AMD3100) on HSPC mobilization in mice.
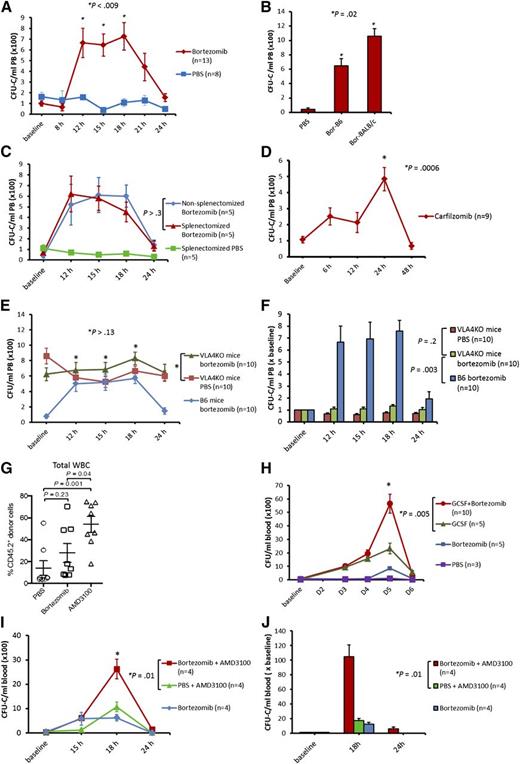
A single intravenous injection of bortezomib resulted in a significant rise in PB colony forming unit-cells (CFU-C) whose numbers peaked at 12 hours and were maintained for at least 6 hours before returning to baseline by 24 hours in both B6 and BALB/c mice (Figure 1A-B). A white blood cell peak of 1.5 ± 0.15-fold over baseline was observed from 12 hours to 15 hours after bortezomib administration. There was no difference in HSPC mobilization by bortezomib in nonsplenectomized mice and splenectomized mice (Figure 1C), suggesting that bortezomib mobilizes HSPCs from the bone marrow rather than from the spleen. We also hypothesized that HSPC mobilization by bortezomib may be a general effect of proteasome inhibition. A single intravenous injection of carfilzomib, a second generation proteasome inhibitor, resulted in a significant CFU-C mobilization (approximately sevenfold), but with slightly different kinetics compared with bortezomib (Figure 1D). Additionally, we hypothesized that because proteasome inhibitors downregulate VCAM-1,4,7 bortezomib should consequently mobilize HSPCs by modulating the VLA-4/VCAM-1 axis. To test this hypothesis, we administered bortezomib to VLA-4KO mice and measured its effect on HSPC mobilization. VLA-4KO mice have a consistently high PB CFU-C ranging from 400/mL to 700/mL (compared with ∼50/mL in B6 mice).8 VLA-4KO mice in our experiments had on average 700/mL PB CFU-C in the resting state. When we mobilized VLA-4KO mice with bortezomib, the number of PB CFU-C observed after treatment with bortezomib was similar to that observed with PBS (Figures 1E-F). These data suggest that bortezomib mobilizes HSPCs by modulation of the VLA-4/VCAM-1 axis. The multilineage engraftment of HPSCs mobilized by bortezomib was assessed using standard competitive repopulating assays, which demonstrated a trend toward increased engraftment in the bortezomib group compared with the PBS group (Figure 1G). In light of modest mobilization effect of bortezomib alone compared with G-CSF or AMD-3100, it is not surprising that the effect of bortezomib was not statistically significant. Additionally we used B6 mice known to be poor mobilizers compared with other strains such as BALB/c.
Mobilization of HSPCs by bortezomib alone or in combination with G-CSF and AMD3100 in mice. Mice were analyzed for PB CFU-Cs at various time points. (A) B6 mice were treated with bortezomib (0.8 mg/kg, intravenously) or phosphate-buffered saline (PBS). Mice were analyzed for PB CFU-Cs at baseline, 8 hours, 12 hours, 15 hours, 18 hours, 21 hours, and 24 hours (combination of 3 experiments; 3 mice in first experiment, 5 mice each in subsequent experiments). (B) Bortezomib (0.8 mg/kg IV) in B6 (Bor-B6), bortezomib (0.8 mg/kg IV) in BALB/c (Bor-BALB/c), and PBS in B6 and BALB/c (PBS). Mice were analyzed for PB CFU-Cs 15 hours after administration of PBS or bortezomib. Bor-BALB/c resulted in higher CFU-C mobilization compared with Bor-B6. (C) Splenectomized and nonsplenectomized (wild-type) B6 mice receiving bortezomib (0.8 mg/kg IV) at baseline. Splenectomized B6 mice that received PBS at baseline were used as a control. (D) Carfilzomib (5 mg/kg IV) was given to B6 mice at baseline and PB CFU-Cs were analyzed at various time points (combination of 2 experiments; 4 mice per arm in first experiment, 5 mice per arm in the second experiment). (E-F) Bortezomib (0.8 mg/kg IV) was given to VLA-4KO and wild-type B6 mice. VLA-4KO mice receiving PBS at baseline were used as control (combination of 2 experiments; 5 mice in each arm per experiment). (G) Competitive repopulation assay. Lethally irradiated CD45.1+ mice received transplants of 2 × 105 congenic CD45.1+/CD45.2+ bone marrow competitor cells plus PBMCs (1.5-3 mL PB) from CD45.2+ mice mobilized with bortezomib, PBS, or AMD3100. PB was harvested 15 hours after injection for bortezomib and PBS, and 3 hours after injection for AMD3100. The contribution of mobilized cell populations to hematopoiesis was determined by flow cytometry for CD45.2+ donor cells 2 months after transplant (combination of 2 experiments; 4 mice in each arm per experiment). (H) Bortezomib (0.8 mg/kg IV) in combination with G-CSF (250 µg/kg, subcutaneously). The G-CSF + bortezomib group received G-CSF on days 1 (baseline), 2, 3, and 4, and bortezomib on day 4. The G-CSF control group received G-CSF on days 1 (baseline), 2, 3, and 4. The bortezomib and PBS control groups received bortezomib (0.8 mg/kg IV) and PBS, respectively, on day 4. Mean PB CFU-C in G-CSF + bortezomib vs G-CSF alone: 5600/mL vs 2300/mL, respectively (P = .005). (I-J) The bortezomib + AMD3100 group received bortezomib (0.8 mg/kg IV) at baseline and AMD3100 (5 mg/kg subcutaneously) 15 hours later. The PBS + AMD3100 group received PBS at baseline followed by AMD3100 15 hours later. The bortezomib control group received bortezomib at baseline. Mice were analyzed for PB CFU-Cs at baseline, 15 hours, 18 hours, and 24 hours. Mean PB CFU-C in bortezomib + AMD3100 vs PBS + AMD3100 at 18 hours: 2600/mL vs 1100/mL, respectively (P = .01). Fold increase in CFU-C over baseline in bortezomib + AMD3100 group vs PBS + AMD3100 group: 105 ± 16 vs 17 ± 3, respectively (P = .01). Data are mean ± standard error.
Mobilization of HSPCs by bortezomib alone or in combination with G-CSF and AMD3100 in mice. Mice were analyzed for PB CFU-Cs at various time points. (A) B6 mice were treated with bortezomib (0.8 mg/kg, intravenously) or phosphate-buffered saline (PBS). Mice were analyzed for PB CFU-Cs at baseline, 8 hours, 12 hours, 15 hours, 18 hours, 21 hours, and 24 hours (combination of 3 experiments; 3 mice in first experiment, 5 mice each in subsequent experiments). (B) Bortezomib (0.8 mg/kg IV) in B6 (Bor-B6), bortezomib (0.8 mg/kg IV) in BALB/c (Bor-BALB/c), and PBS in B6 and BALB/c (PBS). Mice were analyzed for PB CFU-Cs 15 hours after administration of PBS or bortezomib. Bor-BALB/c resulted in higher CFU-C mobilization compared with Bor-B6. (C) Splenectomized and nonsplenectomized (wild-type) B6 mice receiving bortezomib (0.8 mg/kg IV) at baseline. Splenectomized B6 mice that received PBS at baseline were used as a control. (D) Carfilzomib (5 mg/kg IV) was given to B6 mice at baseline and PB CFU-Cs were analyzed at various time points (combination of 2 experiments; 4 mice per arm in first experiment, 5 mice per arm in the second experiment). (E-F) Bortezomib (0.8 mg/kg IV) was given to VLA-4KO and wild-type B6 mice. VLA-4KO mice receiving PBS at baseline were used as control (combination of 2 experiments; 5 mice in each arm per experiment). (G) Competitive repopulation assay. Lethally irradiated CD45.1+ mice received transplants of 2 × 105 congenic CD45.1+/CD45.2+ bone marrow competitor cells plus PBMCs (1.5-3 mL PB) from CD45.2+ mice mobilized with bortezomib, PBS, or AMD3100. PB was harvested 15 hours after injection for bortezomib and PBS, and 3 hours after injection for AMD3100. The contribution of mobilized cell populations to hematopoiesis was determined by flow cytometry for CD45.2+ donor cells 2 months after transplant (combination of 2 experiments; 4 mice in each arm per experiment). (H) Bortezomib (0.8 mg/kg IV) in combination with G-CSF (250 µg/kg, subcutaneously). The G-CSF + bortezomib group received G-CSF on days 1 (baseline), 2, 3, and 4, and bortezomib on day 4. The G-CSF control group received G-CSF on days 1 (baseline), 2, 3, and 4. The bortezomib and PBS control groups received bortezomib (0.8 mg/kg IV) and PBS, respectively, on day 4. Mean PB CFU-C in G-CSF + bortezomib vs G-CSF alone: 5600/mL vs 2300/mL, respectively (P = .005). (I-J) The bortezomib + AMD3100 group received bortezomib (0.8 mg/kg IV) at baseline and AMD3100 (5 mg/kg subcutaneously) 15 hours later. The PBS + AMD3100 group received PBS at baseline followed by AMD3100 15 hours later. The bortezomib control group received bortezomib at baseline. Mice were analyzed for PB CFU-Cs at baseline, 15 hours, 18 hours, and 24 hours. Mean PB CFU-C in bortezomib + AMD3100 vs PBS + AMD3100 at 18 hours: 2600/mL vs 1100/mL, respectively (P = .01). Fold increase in CFU-C over baseline in bortezomib + AMD3100 group vs PBS + AMD3100 group: 105 ± 16 vs 17 ± 3, respectively (P = .01). Data are mean ± standard error.
Ramirez et al9 demonstrated additive/synergistic HSPC mobilization when BIO5192, a small molecule inhibitor of VLA-4, was used in combination with G-CSF or AMD3100.9 Therefore, we hypothesized that bortezomib could augment G-CSF and AMD3100-induced mobilization. Bortezomib enhanced the mobilization effect of G-CSF (Figure 1H) and AMD-3100 (Figure 1I-J) significantly.
In this report, we are the first to show that bortezomib not only directly and rapidly mobilizes HSPCs, but also dramatically enhances the mobilization of HSPCs when given in combination with both G-CSF and AMD3100 in mice by modulating the VLA-4/VCAM-1 axis. The clinical role of bortezomib or other proteasome inhibitors as rapid mobilizing agents in humans is yet to be determined.
Authorship
Acknowledgments: The authors thank Dr Papayannopoulou for providing α4flox/flox mice.
J.F.D. is supported by the National Institutes of Health, National Cancer Institute (R21 CA 141523-01 and R01 CA152329).
Contribution: A.G., M.P.R, L.E., and J.F.D. designed and analyzed the experiments and wrote the paper; A.G., M.P.R., M.L.C., M.S.H., and J.K.R. performed the animal study; and all authors discussed the results and commented on the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John F. DiPersio, Division of Oncology, Department of Medicine, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8007, St Louis, MO 63110; e-mail: jdipersi@dom.wustl.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal