Key Points
IHC is a valuable clinical tool for assessing CD30+ PTCL patients who may respond to CD30-targeting treatment.
CD30 mRNA and protein expression are highly correlated.
Abstract
The extended use of brentuximab-vedotin was reported for CD30+ nonanaplastic peripheral T-cell lymphomas (PTCLs) with promising efficacy. CD30 status assessment is thus a critical factor for therapeutic decision, but the reliability of immunohistochemistry (IHC) in evaluating its expression remains to be defined. This prompted us to investigate the correlation between semiquantitative CD30 protein assessment by IHC and messenger RNA (mRNA) assessment by microarrays in a cohort of 376 noncutaneous PTCLs representative of the main entities. By IHC, CD30 expression was heterogeneous across and within entities and significantly associated with large tumor cell size. In addition to 100% anaplastic large-cell lymphomas, 57% of other PTCL entities were CD30-positive at a 5% threshold. CD30 protein expression was highly correlated to mRNA levels. mRNA levels were bimodal, separating high from low CD30-expressing PTCL cases. We conclude that IHC is a valuable tool in clinical practice to assess CD30 expression in PTCLs.
Introduction
CD30 is a transmembrane receptor with restricted expression on activated T and B cells in normal lymphoid tissues.1,2 In neoplastic conditions, strong CD30 expression is a feature of classical Hodgkin lymphoma and anaplastic large cell lymphomas (ALCL). Given the high response rates seen in phase 2 studies, the novel CD30+ tumor-targeting antibody drug–conjugated brentuximab vedotin (BV) was approved treating relapsed or refractory CD30+ HL and systemic ALCL.3,4
PTCLs compose a group of aggressive neoplasms characterized by unsatisfactory response to multiagent chemotherapy. Recent reports document promising efficacy of BV in nonanaplastic CD30+ PTCLs5,6 ; however, CD30 protein expression in PTCLs is highly heterogeneous among entities,7-16 and the reliability of CD30 status determined by immunohistochemistry (IHC) in the context of prospective BV therapy remains a critical open question. This prompted us to investigate the correlation between CD30 protein expression as evaluated by IHC and messenger RNA (mRNA) expression in a large series of PTCLs to assess if the immunohistochemical scores sufficiently capture variations seen at the mRNA level. We show a consistently significant correlation between mRNA and protein expression for different PTCL entities, supporting that, in clinical practice, IHC is a valuable tool for determining CD30 status in PTCLs.
Study design
Patients and tumor samples
We selected a series of 376 PTCLs with formalin-fixed and frozen samples, diagnosed between 1999 and 2012, from the TENOMIC Consortium Biobank (approved by the local ethics committee, Comité de Protection des Personnes Ile de France 08-009; patients provided informed consent in accordance with the Declaration of Helsinki). In this framework, all cases were reviewed by a panel of expert hematopathologists and diagnosed according to the World Health Organization 2008 criteria. Of these, 238 cases had expression data (Affymetrix HG-U133 plus 2.0 chips, CD30 gene probe set: 206729_at), which include 54 cases from previously reported series.17-19
CD30 expression assessment by combined IHC and gene expression profiling
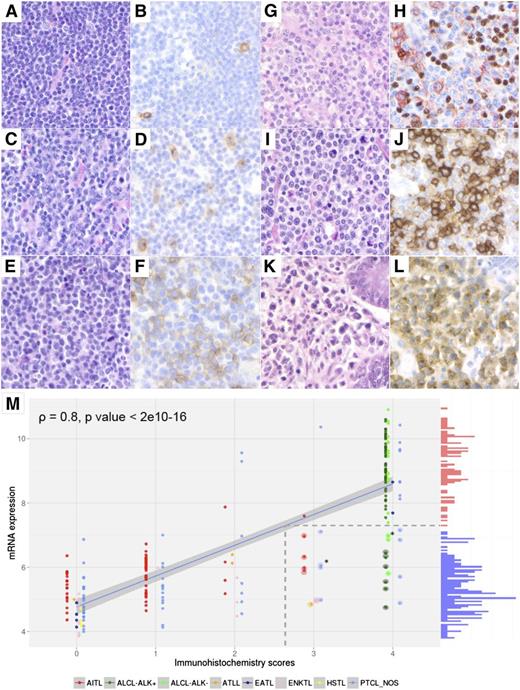
CD30 immunostains, performed on full sections as part of the diagnosis (by the submitting pathologists) or review process or for the purpose of this study (BerH2 antibody on the Ventana automated IHC platform, LYSA-Pathology Laboratory), were scored using a semiquantitative evaluation of the percentage of CD30+ tumor cells on a 5-tiered scale (IHC scores: score 0, <5% of CD30+ tumor cells; score 1, 5% to 24%; score 2, 25% to 49%; score 3, 50% to 75%; score 4, >75%; Figure 1A-F). To score CD30 expression in tumor cells only, CD30 immunostains were compared with the immunostains for CD3 and/or other relevant T-cell markers and CD20, especially in angioimmunoblastic T-cell lymphoma (AITL). CD30 staining intensity was also graded as weak, moderate, or strong. Cytological features, recorded as the proportion of small, medium, and large cells, were collapsed to 2 categories based on the predominance of the small or large cell component. Ten representative cases of AITL were subjected to double staining for the nuclear B-cell–associated marker PAX5 and CD30 (Figure 1G-H).
Correlation between CD30 mRNA and protein expression by tumor cells assessed by IHC in 238 PTCLs. Representative examples of the CD30 expression scores in PTCLs (A,C,E,G,I,K: hematoxylin and eosin; B,D,F,J,L: CD30 immunostaining by immunoperoxidase; H: double CD30/PAX5 immunostaining by immunoperoxidase, 3-amino-9-ethyl carbazole for CD30, and 3,3′ diaminobenzidine for PAX5, respectively; magnification ×40). (A-B) PTCL-NOS composed of small tumor cells with CD30 immunohistochemical score 0 (rare CD30+ cells, <5%). (C-D) PTCL-NOS composed of small/medium atypical cells with CD30 immunohistochemical score 1 (scattered tumor cells express CD30). (E-F) PTCL-NOS composed of medium/large tumor cells with immunohistochemical score 2. (G-H) AITL featuring a CD30 immunohistochemical score 2 (the CD30+ cells mainly correspond to CD30+/PAX5− tumor cells, in addition to scattered CD30+/PAX5+ B blasts). (I-J) PTCL-NOS composed of medium/large tumor cells with CD30 immunohistochemical score 3. (K-L) EATL type 1 composed of large tumor cells with CD30 immunohistochemical score 4. Correlation between CD30 IHC scores and CD30 mRNA expression levels (M; histogram shown on the y-axis, red bars = high expression; blue bars = low expression). The regression line between mRNA and protein expression values is shown (blue); this line is used to project the mRNA expression value that distinguishes high- from low-CD30–expressing samples onto its corresponding IHC level (broken lines). Samples that express low levels of CD30 mRNA but that have high IHC scores are shown with a halo. Asterisks indicate points where 2 samples overlap.
Correlation between CD30 mRNA and protein expression by tumor cells assessed by IHC in 238 PTCLs. Representative examples of the CD30 expression scores in PTCLs (A,C,E,G,I,K: hematoxylin and eosin; B,D,F,J,L: CD30 immunostaining by immunoperoxidase; H: double CD30/PAX5 immunostaining by immunoperoxidase, 3-amino-9-ethyl carbazole for CD30, and 3,3′ diaminobenzidine for PAX5, respectively; magnification ×40). (A-B) PTCL-NOS composed of small tumor cells with CD30 immunohistochemical score 0 (rare CD30+ cells, <5%). (C-D) PTCL-NOS composed of small/medium atypical cells with CD30 immunohistochemical score 1 (scattered tumor cells express CD30). (E-F) PTCL-NOS composed of medium/large tumor cells with immunohistochemical score 2. (G-H) AITL featuring a CD30 immunohistochemical score 2 (the CD30+ cells mainly correspond to CD30+/PAX5− tumor cells, in addition to scattered CD30+/PAX5+ B blasts). (I-J) PTCL-NOS composed of medium/large tumor cells with CD30 immunohistochemical score 3. (K-L) EATL type 1 composed of large tumor cells with CD30 immunohistochemical score 4. Correlation between CD30 IHC scores and CD30 mRNA expression levels (M; histogram shown on the y-axis, red bars = high expression; blue bars = low expression). The regression line between mRNA and protein expression values is shown (blue); this line is used to project the mRNA expression value that distinguishes high- from low-CD30–expressing samples onto its corresponding IHC level (broken lines). Samples that express low levels of CD30 mRNA but that have high IHC scores are shown with a halo. Asterisks indicate points where 2 samples overlap.
Statistical analyses
Spearman correlation coefficients were calculated between CD30 immunohistochemical scores and mRNA expression. Fisher’s exact test was used to evaluate the significance of cell size and staining intensity association with either CD30− or CD30+ samples. CD30 mRNA expression values were fitted with a 2-component Gaussian mixture model to test for bimodality (bimodality index threshold ≥1.1), and the intersection of the curves was used as the mRNA threshold for CD30 positivity.20
Results and discussion
CD30 protein expression levels per entity are summarized in Table 1. Overall, 66% (248/376) of PTCLs expressed CD30 (score ≥1), but only 34.5% (130/376) showed a high CD30 expression (score ≥3). Except for ALCLs, which were consistently CD30-positive, CD30 protein expression was heterogeneous across and within all PTCL entities. Of 141 PTCL-not otherwise specified (PTCL-NOS), 82 (58%) had CD30 expression scored 1 or higher, and 32 of these (23%) had a score ≥3. Among AITLs, 61/97 (63%) expressed CD30 (score 1 or higher), but only a minority of these (5/97, 5%) showed a score ≥3. Five of 9 cases of adult T-cell leukemia/lymphomas (ATLL) were CD30+, including 1 with a score of 3. Among enteropathy-associated T-cell lymphoma (EATLs), 7/10 of the type I cases were CD30+ (score ≥3) whereas none of 4 type II cases tested had detectable CD30 expression. In extranodal natural killer/T-cell lymphoma, CD30 expression was found in 13/28 of cases, with a score ≥3 in 8 of them. Finally, none of the 7 cases of hepatosplenic T-cell lymphoma expressed CD30. Furthermore, considering nonanaplastic PTCLs, CD30 expression was associated with tumor cell size: cases with higher immunohistochemical scores composed a higher proportion of large-cell tumors (supplemental Figure 1A [available on the Blood Web site], P = 1.2e-10), and small-cell PTCLs were more likely to be CD30-negative, whereas, conversely, a higher proportion of large-cell PTCLs were CD30-positive (96/139, 69.1%; P = 6.3e-05; supplemental Figure 1B). Considering CD30-positive PTCLs, those composed of predominantly large cells tended to display stronger staining intensity than those predominantly composed of smaller cells (P = 5.3e-04; supplemental Figure 1C). In addition, stronger CD30 staining intensities were significantly associated with higher immunohistochemical scores (P = 8.6e-08; supplemental Figure 1D). Altogether, the CD30 immunohistochemical results presented here—on the largest PTCL cohort so far—expand those of previous studies.7-12,14-16,21,22 We found a higher percentage of CD30+ PTCL-NOS than in the study published by Went et al, which was based on tissue microarrays.13 Interestingly, we found a higher percentage of CD30+ cases among AITLs than previously reported,13,16 possibly because of the frequently weak CD30 staining observed in this entity (27/61 cases) (Figure 1; supplemental Figure 1D). The PAX5/CD30 double staining indeed confirmed CD30 expression in PAX5-negative/CD30-positive small- to medium-sized tumor T cells in addition to PAX5-positive CD30-positive large B-cell blasts as classically described in AITL (Figure 1).
CD30 immunohistochemical expression in PTCLs
| % of CD30+ tumor cells . | ALCL ALK+(N = 61) . | ALCL ALK−(N = 19) . | PTCL NOS (N = 141) . | AITL (N = 97) . | ENKTL (N = 28) . | EATL (N = 14) . | ATLL (N = 9) . | HSTL (N = 7) . |
|---|---|---|---|---|---|---|---|---|
| Score 0 | 0 | 0 | 59 | 36 | 15 | 7 | 4 | 7 |
| <5% | 42% | 37% | 53.5% | 50% | 44% | 100% | ||
| Score 1 | 0 | 0 | 37 | 46 | 2 | 0 | 1 | 0 |
| 5-24% | 26% | 47% | 7% | 11% | ||||
| Score 2 | 3 | 0 | 13 | 10 | 3 | 0 | 3 | 0 |
| 25-49% | 5% | 9% | 10% | 11% | 33% | |||
| Score 3 | 1 | 0 | 14 | 5 | 4 | 1 | 1 | 0 |
| 50-75% | 2% | 10% | 5% | 14% | 7% | 11% | ||
| Score 4 | 57 | 19 | 18 | 0 | 4 | 6 | 0 | 0 |
| >75% | 93% | 100% | 13% | 14% | 43% | |||
| Total positive cases (scores 1-4) | 61 | 19 | 82 | 61 | 13 | 7 | 5 | 0 |
| 100% | 100% | 58% | 63% | 46% | 50% | 55.5% | ||
| Strongly positive cases (scores 3-4) | 58 | 19 | 32 | 5 | 8 | 7 | 1 | 0 |
| 95.1% | 100% | 23% | 5% | 28.5% | 50% | 11% |
| % of CD30+ tumor cells . | ALCL ALK+(N = 61) . | ALCL ALK−(N = 19) . | PTCL NOS (N = 141) . | AITL (N = 97) . | ENKTL (N = 28) . | EATL (N = 14) . | ATLL (N = 9) . | HSTL (N = 7) . |
|---|---|---|---|---|---|---|---|---|
| Score 0 | 0 | 0 | 59 | 36 | 15 | 7 | 4 | 7 |
| <5% | 42% | 37% | 53.5% | 50% | 44% | 100% | ||
| Score 1 | 0 | 0 | 37 | 46 | 2 | 0 | 1 | 0 |
| 5-24% | 26% | 47% | 7% | 11% | ||||
| Score 2 | 3 | 0 | 13 | 10 | 3 | 0 | 3 | 0 |
| 25-49% | 5% | 9% | 10% | 11% | 33% | |||
| Score 3 | 1 | 0 | 14 | 5 | 4 | 1 | 1 | 0 |
| 50-75% | 2% | 10% | 5% | 14% | 7% | 11% | ||
| Score 4 | 57 | 19 | 18 | 0 | 4 | 6 | 0 | 0 |
| >75% | 93% | 100% | 13% | 14% | 43% | |||
| Total positive cases (scores 1-4) | 61 | 19 | 82 | 61 | 13 | 7 | 5 | 0 |
| 100% | 100% | 58% | 63% | 46% | 50% | 55.5% | ||
| Strongly positive cases (scores 3-4) | 58 | 19 | 32 | 5 | 8 | 7 | 1 | 0 |
| 95.1% | 100% | 23% | 5% | 28.5% | 50% | 11% |
ENKTL, extranodal natural killer/T-cell lymphoma; HSTL, hepatosplenic T-cell lymphoma.
For 238 PTCLs with IHC and microarray profiles, CD30 immunohistochemical scores were significantly correlated with CD30 mRNA levels (ρ = 0.8, P < 2e10−16) (Figure 1M). Similar results were found when excluding ALCL patients (n = 158, ρ = 0.60, P < 2e10−16), by considering only PTCL-NOS (n = 65, ρ = 0.65, P = 5.68e10−9) (supplemental Figure 2), or only AITL patients (n = 64, ρ = 0.44, P = 3.17e10−4)(not shown). However, a minority of cases (n = 24/238, 10%), including 10 ALCLs, 9 PTCL-NOSs, 4 AITLs, and 1 ATLL, showed a discordant profile with a high CD30 IHC score (≥3) but low CD30 mRNA levels (Figure 1). These apparent discrepancies can be partly attributed to (1) PTCLs with a high proportion of CD30+ tumor cells, but featuring weak staining intensity (7/24); (2) samples with a low tumor cell content (2/24); or (3) morphological variants of ALK+ ALCL (4/24). Conversely, all cases with high mRNA expression levels had detectable CD30 protein with an immunohistochemical score ≥2. Altogether, these findings indicate that IHC is a sensitive method to assess CD30 expression.
Furthermore, CD30 mRNA expression was found to be bimodal and permits the identification of 2 groups of PTCLs: 1 group representing 66% of the cases expressing low levels of CD30 mRNA and a second group (34% of the cases) expressing high levels of CD30 mRNA corresponding to CD30 protein expression threshold between IHC scores 2 and 3 (Figure 1M). A bimodal distribution of CD30 mRNA expression levels was also observed when excluding the ALCLs or when considering PTCL-NOS only (supplemental Figure 2A-B). The biological significance and therapeutic impact of CD30 bimodality warrant further studies.
Considering recent studies that show no apparent correlation between CD30 expression by tumor cells and BV response in lymphomas such as nonanaplastic PTCLs and diffuse large B-cell lymphomas as well as reported remissions in CD30-negative or weakly CD30-positive lymphomas,6,23 the mechanisms determining BV antitumor activity remain unclear in these neoplasms. A possible hypothesis is that the drug diffuses from the targeted CD30+ reactive cells to the tumor microenvironment and causes cytotoxicity on bystander tumor cells.24 Given the presence of CD30+ reactive B blasts in AITL, we reevaluated 24 of the CD30-negative AITL (score 0) by including CD30+ B blasts, and 16 of them (66%) were reclassified as score 1. Further studies are needed to deepen the influence of combined CD30 expression levels from the tumor and its microenvironment in the therapeutic response to BV.
In conclusion, our study establishes for the first time a significant correlation between CD30 mRNA and protein expression, assessed by IHC, across different PTCL entities. We also show that IHC adequately captures CD30 mRNA expression features—namely heterogeneity and bimodality of CD30 expression in PTCLs. These findings indicate that IHC performed in a routine clinical setting is a valuable, practical tool to assess CD30 expression in PTCLs. Based on a large series of cases, we show that around 60% of AITL and PTCL-NOS patients and around 50% of patients with extranodal PTCLs have detectable CD30 expression using a 5% threshold. From a clinical perspective, this suggests that the majority of PTCLs may be potential candidates for CD30-targeting strategies, although further large clinical trials correlating response to BV and CD30 expression levels are needed to determine the criteria for patient eligibility.
This study was presented in part at the 13th International Conference on Malignant Lymphoma (Lugano, Switzerland).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Caroline Communaux and Nadine Vailhen from the LYSA-Pathology for their technical and logistical assistance, and Virginie Fataccioli for the coordination of the TENOMIC program.
This work was supported by grants from the MEDIC Foundation, the Ligue du Cancer (Switzerland), the Institut National du Cancer, the Fondation pour la Recherche Médicale, the Programme Hospitalier de Recherche Clinique (France), and the Plan Cancer (Belgium).
Authorship
Contribution: P.G. and L.d.L. designed and supervised the study; C.B., P.G., and L.d.L. reviewed all the cases for CD30 immunohistochemical expression; M.P.D. performed the statistical analysis; E.M. and M.D. supervised the statistical analysis; C.B., M.P.D., E.M., M.D., P.G., and L.d.L. analyzed and interpreted the results and wrote the paper; M.P., B.F., and A.M. participated in the pathologic review of patients entered in the TENOMIC consortium; C.H., R.D., and O.T. participated in documenting the patients; and all other authors contributed data and approved the paper.
Conflict-of-interest disclosure: C.B., C.H., R.D., and P.G. have acted on the advisory board for TAKEDA France. R.D. is a member of an independent data and safety monitoring committee for a clinical trial sponsored by TAKEDA. The remaining authors declare no competing financial interests.
Correspondence: Laurence de Leval, Institut de Pathologie, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland; e-mail: laurence.deleval@chuv.ch.
References
Author notes
C.B. and M.P.D. contributed equally to this study.
P.G. and L.d.L. codirected this study.