In this issue of Blood, Krysov et al demonstrate that B-cell receptor (BCR) signaling in chronic lymphocytic leukemia (CLL) results in partial activation of the unfolded protein response (UPR).1
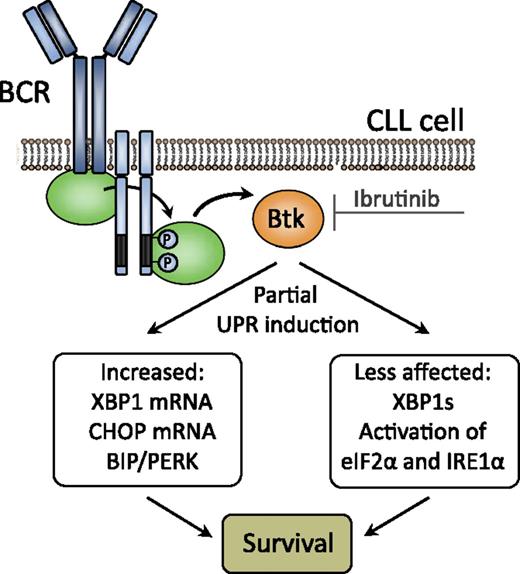
BCR signaling through BTK (which is targeted by the small molecule inhibitor ibrutinib) induces partial UPR activation in CLL, resulting in increased CLL cell survival. XBP1s, XBP1 active splice variant.
BCR signaling through BTK (which is targeted by the small molecule inhibitor ibrutinib) induces partial UPR activation in CLL, resulting in increased CLL cell survival. XBP1s, XBP1 active splice variant.
CLL is characterized by the accumulation of a monoclonal population of malignant B cells with a typical CD5+CD23+CD20dim surface Igdim immunophenotype that is refractory to apoptosis. CLL is a heterogeneous disease in which, in addition to the accumulation of mutations and epigenetic changes, microenvironmental factors, in particular antigenic stimulation via the BCR, are thought to contribute to leukemogenesis. Several lines of evidence support a role for antigenic stimulation in CLL pathogenesis. First, CLL patients with hypermutated immunoglobulin heavy chain V (IGHV) genes have a more favorable prognosis than those with unmutated IGHV genes, who tend to show progressive disease and resistance to therapy. Second, there are CLL patient subgroups with almost identical, stereotyped BCRs, for several of which antigens have been identified over the years. Third, CLL cells readily upregulate surface immunoglobulin M (IgM) expression in vitro, once they are isolated and thus deprived of their putative in vivo ligands. In contrast with these findings supporting the initiation of BCR signaling by exogenous antigen, Dühren-von Minden et al2 provided evidence for a role of autonomous BCR signaling in CLL, through binding of CLL-derived BCR to a conserved region within IgH framework region 2. In either model, it appears that BCR signaling induces a state of functional anergy or cellular unresponsiveness. The work by Krysov et al now supports a role for BCR signaling in CLL cell survival through induction of a partial UPR.
The UPR is a multifunctional response pathway that signals from the endoplasmic reticulum (ER) to the nucleus and can promote cell survival or cell death, dependent on the extent or duration of the activating signal.3 It is typically initiated in response to the accumulation of unfolded proteins or elevated secretory protein synthesis within the ER lumen. A classic example is UPR induction during plasma cell differentiation, which anticipates ER stress prior to enhanced immunoglobulin production. Progressive development of the ER and the Golgi apparatus in CLL was first documented >30 years ago.4,5 However, because CLL cells do not manifest a prominent expansion of their ER as typically found in the plasma cell malignancy multiple myeloma, the functional roles of the ER stress-response proteins in CLL remained largely unexplored. Several findings prompted Krysov et al to study the UPRT in human CLL: (1) BCR signaling-induced UPR is not limited to cells in which BCR signaling concomitantly induces activation6 ; (2) CLL cells can express components of the UPR pathway; and (3) pharmacological inducers of high level UPR activation promote apoptosis of CLL cells. They show that in circulating CLL cells the basal activation of the UPR was increased compared with normal B cells.1 RNA levels of the UPR transcription factors C/EBP-homologous protein (CHOP) and X-box protein 1 (XBP1) were correlated with surface IgM signaling capacity and with disease aggressiveness. In addition, BCR stimulation in vitro increased the expression of UPR components, which could effectively be blocked by small molecule inhibitors of BCR signaling kinases, including the Bruton's tyrosine kinase (BTK) inhibitor ibrutinib. Finally, relatively high levels of UPR components were found in lymph node proliferation centers in vivo. These findings indicate that UPR activation promotes survival and contributes to the growth promoting effects of BCR signaling in CLL (see figure). Thus, the successful antitumor activity of ibrutinib recently observed in clinical studies may be partly based on inhibition of UPR activation.
Importantly, the authors found that UPR activation in CLL is only partial: whereas expression of CHOP and XBP1 RNA and binding immunoglobulin protein (BIP) were increased, activation of protein kinase RNA-like ER kinase (PERK) was substantial, and phosphorylation of eukaryotic translation initiator factor 2α (eIF2α) was limited (see figure). Also, processing of unspliced XBP1 to functional XBP1s by the UPR stress sensor inositol-requiring protein 1α (IRE1α) was not detectable. A similar partial activation of UPR components, characterized by upregulation of ER chaperones and CHOP and minimal XBP1 splicing, was found during normal B-cell differentiation. Such anticipatory UPR is thought to prepare B cells for subsequent immunoglobulin secretion.6,7 Most likely, the partial UPR activation in CLL supports cell survival, because IRE1α, the principal mediator of UPR-associated apoptosis, is not effectively activated in CLL cells. Moreover, the expression of BIP, a chaperone with prosurvival function, is increased.
Indeed, it has been reported that the ER stress response is critical for the growth of human CLL and mouse CLL (in the mouse model with overexpression of the TCL1 gene).8,9 Based on findings showing that inhibitors of the ER stress response induce apoptosis of mouse and human CLL cells in vitro and suppressed leukemic progression in mice in vivo, the UPR pathway may be a powerful novel molecular target for the treatment of CLL patients.7,8 In this context, an inhibitor of the IRE1α RNase activity has been developed, which effectively induces leukemic regression in CLL-bearing TCL1 transgenic mice.9 Apart from their potential as a treatment strategy, UPR components are expected to have value as prognostic or predictive markers, because higher RNA expression levels of CHOP and XBP1 were correlated with more aggressive disease.
On the basis of its profound antitumor activity,10 the BTK inhibitor ibrutinib was recently registered in the United States for previously treated patients with CLL. Interestingly, Krysov et al found that treatment of CLL cells in vitro with small molecule inhibitors of BTK and spleen tyrosine kinase reduced the expression of anti-IgM–induced BIP and PERK. It is therefore conceivable that the successful antitumor activity of ibrutinib is partly based on inhibition of UPR activation. It will be interesting to investigate whether its in vitro effects on BIP and PERK expression can be used to monitor or predict response or resistance to ibrutinib. Conversely, because inhibition of IRE1α RNase activity synergized with ibrutinib to induce apoptosis in human CLL cells,9 it would be worthwhile to explore the effects of UPR inhibition with ibrutinib in a combination treatment strategy.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal