Abstract
Minimal residual disease (MRD) assessment provides early evaluation of the efficacy of anti-leukemic therapy. Previous studies suggested that MRD status serves as a prognostic tool in AML. By contrast to pediatric ALL, where assessment of MRD routinely informs prognosis and guides therapeutic decisions, MRD in adult AML is not routinely used in the clinical context. Moreover, the relationship of MRD status to state-of-the-art molecular prognostic algorithms has not been investigated; it is unknown whether integrating MRD data with molecular profiling allows investigators to better identify patients at increased risk of relapse. In order to investigate the clinical relevance of MRD and its relationship with molecular-based prognostic schema, we performed flow cytometric MRD profiling on AML patients enrolled in the ECOG-led intergroup phase 3 trial, E1900, which randomized patients to induction with cytarabine plus 45 or 90mg/m2 daunorubicin.
189 patients (29.3% of the original cohort) had MRD analyzed at morphologic and hematologic complete remission (CR). MRD was evaluated by 4-color flow cytometry (FC) and positivity defined as 0.1% of leukemic cells in bone marrow. There was no association between MRD status and daunorubicin dose (p=1.0), suggesting that dose-intensification, which significantly improving overall survival (OS) (Fernandez et al, NEJM 2010), did not reduce the frequency of MRD positivity. We found a highly significant relationship between MRD positivity and OS (p=0.0002). Of note, the relevance of MRD status to OS was significant in univariate (p=0.0002) and multivariate analyses, after adjusting for age, gender, Hb, WBC/platelet count and cytogenetic risk profile, with a hazard ratio (HR) of MRD positivity vs. MRD negativity of 2.12 (95% CI, 1.31 to 3.41; p=0.0022). The impact of MRD status after consolidation on disease-free survival (DFS) and OS was assessed in 140 patients with evaluable samples at completion of consolidation. Both univariate and multivariate analyses showed strong association between MRD positivity and DFS (p<0.0001 in both analyses) and OS (p<0.0001 in both analyses); the HR for multivariate analysis was 4.16. Notably, using different cut-off thresholds for defining MRD positivity (0.01% and 0.05%) did not change the results of these analyses, confirming that the clinically relevant MRD level in adult AML is 10-times higher than in pediatric ALL.
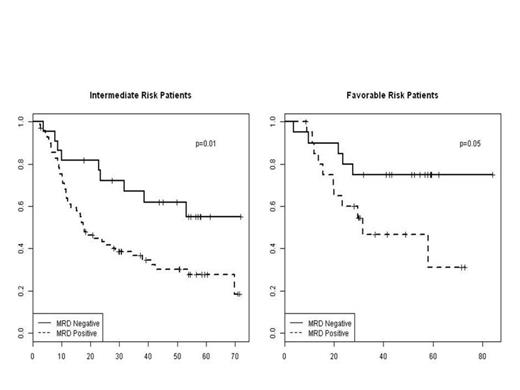
We next investigated whether MRD status provided prognostic information independent of integrated molecular profiling. We assessed the prognostic relevance of MRD status in each of our previously reported molecularly defined risk categories (Patel et al, NEJM 2012). The frequency of MRD positivity increased with increasing risk of relapse as assessed by integrated molecular profiling (p=0.017), with 51% of favorable patients, 76% of intermediate patients, and 73% of unfavorable risk patients having evidence of MRD at the time of CR. Multivariate analysis demonstrated that MRD status had a powerful impact on OS independent of molecularly defined risk (p=0.003), demonstrating that molecular analysis of pre-therapy leukemic cells and post-induction analysis of MRD status independently predict outcome in AML. Notably, the impact of MRD status on outcome was most dramatic in favorable and intermediate risk patients; in each of these subgroups MRD positive patients had an absolute reduction in survival by 28-30% compared to MRD negative patients. By contrast, MRD positive patients with molecularly defined adverse risk did not fare worse than MRD negative patients of this risk class, suggesting that patients with high risk genetic lesions have poor OS regardless of MRD status post induction therapy.
The presence of MRD, assessed by flow cytometry, was an independent prognostic factor in newly diagnosed AML patients, both at time of CR and following consolidation. MRD had a strong association with OS even after adjusting for cytogenetic and molecular abnormalities with a most powerful impact on outcome in patients with molecularly defined favorable and intermediate risk disease. Subsequent studies are waranted to delineate the molecular basis for MRD in different AML subsets, and to investigate if MRD-directed therapies can improve outcomes by reducing disease relapse.
Association between MRD and OS, after adjusting for Integrated Genetic Risk Stratification
Association between MRD and OS, after adjusting for Integrated Genetic Risk Stratification
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal