Abstract
Background: IDA is common in cancer patients due to blood loss and inflammation. Many do not tolerate oral iron or adequately respond. Two Phase-3 trials investigated the efficacy and safety of IV FER for treatment of IDA in patients with a history of unsatisfactory oral iron therapy or in whom oral iron could not be used. This analysis explored the impact of IV iron treatment on the subgroup of patients with cancer.
Methods: Data from patients with cancer were extracted from 2 RCTs (NCT01114139; NCT01114204) in which patients with baseline (BL) Hgb 7-10g/dL, TSAT <20%, were randomized 3:1 to FER (510mg, 2 injections, over 2-8 days) or placebo (PL), and 2:1 to FER or 1g iron sucrose (IS, 200mg, 5 doses over 14 days). Laboratory and safety data were pooled for this analysis. Differences within groups in change from BL Hgb were analyzed with paired t-test, and between groups were explored with ANCOVA adjusted for BL (missing values imputed as 0 change).
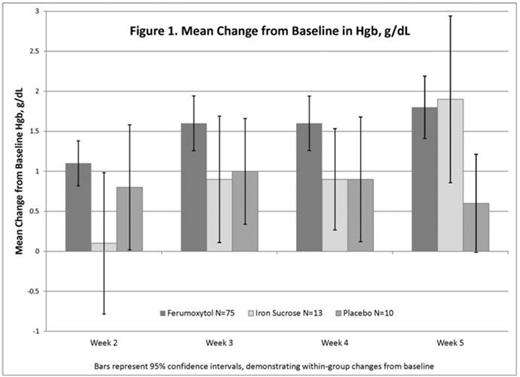
Results: The ITT population of all 1413 patients who were exposed to study drug included 98 patients with cancer (median age 60.5, 71% female, 65% white, BL TSAT 9.2±9.7%, ferritin 121 ±187ng/mL). Gastrointestinal cancers were most common (42), followed by breast (14), cervix (10), and lung (9). During the studies, 45 patients received chemotherapy, 19 with platinum-based regimens. ESA doses were neither increased >20% nor initiated in any treatment group. Transfusions were received by 3 (23%) of IS and 6 (8%) of FER patients. Treatment with both IV irons was associated with a significant increase in Hgb by end of study (FER 1.8 g/dL, p<0.0001; IS 1.9 g/dL, p=0.002; Figure 1).
However, the response with FER occurred earlier, with a significantly greater increase than IS at week 2 (1.1 vs 0.1, p= 0.007) and greater than PL at week 5 (1.8 vs 0.5 g/dL, p=0.02) (LS means). A summary of the frequency and severity of adverse events among the cancer patients subgroup and the full study populations are provided in Table 1.
Adverse Event Summary
| . | Cancer Patients Subgroup . | Pooled Overall Study Population . | ||||
|---|---|---|---|---|---|---|
| Adverse Event (AE) Category, Subjects n(%) | Ferumoxytol N=75 | Iron Sucrose N=13 | Placebo N=10 | Ferumoxytol N=1014 | Iron Sucrose N=199 | Placebo N=200 |
| All AEs | 48 (64.0) | 6 (46.2) | 8 (80.0) | 467 (46.1) | 88 (44.2) | 86 (43.0) |
| Related AEs (1) | 6 (8.0) | 0 | 1 (10.0) | 147 (14.5) | 32 (16.1) | 15 (7.5) |
| Serious AEs | 7 (9.3) | 1 (7.7) | 1 (10.0) | 33 (3.3) | 5 (2.5) | 6 (3.0) |
| Related Serious AEs | 2 (2.7) | 0 | 0 | 6 (0.6) | 0 | 0 |
| AEs of Special Interest (2) | 4 (5.3) | 1 (7.7) | 0 | 33 (3.3) | 10 (5.0) | 2 (1.0) |
| AEs Resulting in Study Discontinuation | 0 | 0 | 0 | 6 (0.6) | 2 (1.0) | 2 (1.0) |
| Death (3) | 1 (1.3) | 0 | 1 (10.0) | 3 (0.3) | 0 | 1 (0.5) |
| (1) Classified by investigator as related to study drug. (2) Protocol defined – including hypotension and hypersensitivity (3) Assessed as unrelated by the Investigator | ||||||
| . | Cancer Patients Subgroup . | Pooled Overall Study Population . | ||||
|---|---|---|---|---|---|---|
| Adverse Event (AE) Category, Subjects n(%) | Ferumoxytol N=75 | Iron Sucrose N=13 | Placebo N=10 | Ferumoxytol N=1014 | Iron Sucrose N=199 | Placebo N=200 |
| All AEs | 48 (64.0) | 6 (46.2) | 8 (80.0) | 467 (46.1) | 88 (44.2) | 86 (43.0) |
| Related AEs (1) | 6 (8.0) | 0 | 1 (10.0) | 147 (14.5) | 32 (16.1) | 15 (7.5) |
| Serious AEs | 7 (9.3) | 1 (7.7) | 1 (10.0) | 33 (3.3) | 5 (2.5) | 6 (3.0) |
| Related Serious AEs | 2 (2.7) | 0 | 0 | 6 (0.6) | 0 | 0 |
| AEs of Special Interest (2) | 4 (5.3) | 1 (7.7) | 0 | 33 (3.3) | 10 (5.0) | 2 (1.0) |
| AEs Resulting in Study Discontinuation | 0 | 0 | 0 | 6 (0.6) | 2 (1.0) | 2 (1.0) |
| Death (3) | 1 (1.3) | 0 | 1 (10.0) | 3 (0.3) | 0 | 1 (0.5) |
| (1) Classified by investigator as related to study drug. (2) Protocol defined – including hypotension and hypersensitivity (3) Assessed as unrelated by the Investigator | ||||||
Conclusions: These results indicate that cancer patients with IDA, who had not tolerated or responded to oral iron, responded to both IV FER and IS with a significant increase in Hgb. The response to IV FER appeared earlier. However,given the relatively small N, limited conclusions can be made, and larger studies will be needed to further define the responses.
Vadhan-Raj:AMAG Pharmaceuticals, Inc.: Research Funding. Off Label Use: Both of the study drugs, ferumoxytol and iron sucrose, are only approved for the treatment of iron deficiency anemia in patients with chronic kidney disease.. Dahl:AMAG Pharmaceuticals, Inc.: Employment, Equity Ownership. Bernard:AMAG Pharmaceuticals, Inc.: Employment, Equity Ownership. Li:AMAG Pharmaceuticals, Inc.: Employment, Equity Ownership. Strauss:AMAG Pharmaceuticals, Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal