Abstract
BACKGROUND AND AIMS: Hairy cell leukemia (HCL) is very sensitive to purine analogs (PAs), but ~40% of patients relapse and become progressively less responsive to these myelotoxic and immune-suppressive drugs. Having discovered the BRAF-V600E kinase-activating mutation as the genetic lesion underlying HCL (Tiacci et al, NEJM 2011;364:2305), we performed the first clinical trial of a BRAF inhibitor (vemurafenib) in refractory/relapsed HCL. In particular, this is a phase-2, academic, single-arm, Italian, multi-center (n=8) study (HCL-PG01; EudraCT 2011-005487-13).
METHODS: In 11 months we enrolled 28 BRAF-V600E+ HCL patients, needing therapy due to cytopenias and including: i) 6 patients primary refractory to a PA; ii) 21 patients who relapsed early and/or repeatedly after PAs and had received a median of 4 previous therapies; and iii) a 81-year old patient showing severe myelotoxicicity after a PA (discouraging its further use). Previous treatments other than PAs included interferon, rituximab and splenectomy in 12, 14 and 8 patients, respectively. Complete remission (CR) required resolution of cytopenias (N≥1500/mmc, PLT≥100000/mmc, Hb≥11 g/dl), no morphological evidence of HCL cells in the bone marrow biopsy and blood smear, and no splenomegaly. Partial remission (PR) required resolution of cytopenias, and a ≥50% reduction of splenomegaly and of marrow and blood HCL involvement by immunophenotyping. Two patients were not evaluable as they went off-study after ≤1 week of treatment (due to drug-unrelated acute myocardial infarction and consent withdrawal after grade-3 drug-related reversible pancreatitis).
RESULTS: Vemurafenib, given orally at the dose of 960 mg twice daily on an outpatient basis for a median of 16 weeks, was generally well tolerated. Drug-related adverse events (mainly arthralgias, skin toxicities, pancreatitis; no myelosuppression) were frequent, but reversible in all patients, and were typically grade 1-2. Only 7 patients developed grade 3 events, and none grade 4 events. Although we did not observe any cutaneous squamous cell carcinomas/keratoachantomas (as reported in BRAF-V600E+ melanoma patients treated with vemurafenib), 3 patients developed 2 basaliomas and 1 superficial melanoma, all treated with a simple excision.
Notably, overall response rate was 96% (25/26 patients): 9/26 (34.6%) CRs and 16/26 (61.4%) PRs, obtained after a median of 8 and 9 weeks respectively. CR and PR patients included 1 and 5 primary refractory ones, respectively, as well as 4 and 10 not responding to the last prior treatment, respectively.
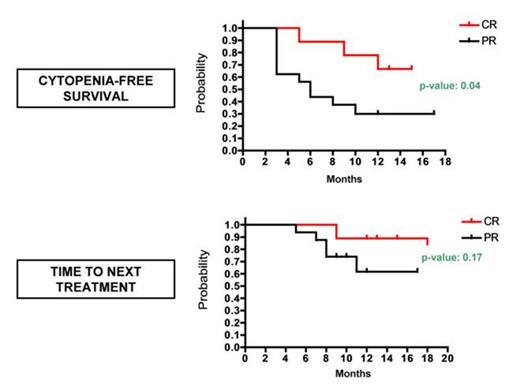
In all CR patients immunohistochemistry showed minimal residual disease (≤10%) at the end of treatment. Six of 9 (67%) CR patients enjoyed normal blood counts at a median of 13 (range 12-15) months from the end of treatment (see Figure): 3 of these 6 patients showed no morphological evidence of HCL in the bone marrow biopsy (complying with a continuous CR) at 12, 13 and 15 months, respectively, whereas the other 3 lost the bone marrow CR status, all at 12 months. The remaining 3/9 CR patients (33%) developed a mild cytopenia (N ~1000/mmc or PLT ~80000/mmc) 5, 9 and 12 months post-treatment, respectively: in the 2nd patient the cytopenia remained stable until the last follow-up at 15 months, whereas in the other two cases it worsened requiring therapy 9 and 18 months post-treatment, respectively (see Figure). These two latter patients were recently retreated with vemurafenib for 12 and 4 weeks, and obtained a PR and a second CR.
Among the 16 PR patients, 5 (31%) mantain normal blood counts at a median of 12 (range 8-17) months post-treatment (see Figure). The other 11 PR patients developed cytopenia(s) after 3 months of median follow-up (range 5-10): in 6 patients (38%) no anti-leukemic therapy was started at a median of 9 (range 6-12) months post-treatment, whereas in the remaining 5 cases (31%) cytopenia(s) worsened requiring therapy at a median of 8 (range 5-11) months of follow-up (see Figure). Four of these latter 5 patients were retreated with vemurafenib for 12 weeks: 3 cases had a minor response and the last one witnessed a second PR that lasted less than the first PR (3 versus 9 months).
CONCLUSIONS: In heavily pre-treated HCL patients, a short oral course of vemurafenib was safe, and proved quickly and highly active. Retreatment with vemurafenib was able to reinduce remissions in patients relapsing after a CR, but was less effective in patients relapsing after a PR.
Off Label Use: Off-label use of vemurafenib in hairy cell leukemia will be discussed as part of a clinical research protocol..
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal