Abstract
Background: Among the most serious complications of hemophilia A is anti-VIII inhibitor formation, which occurs in ~25% of patients, typically in childhood within the first 20 exposures. Morbidity is high, hospitalization is frequent, and healthcare cost is high. Thus, immune tolerance induction (ITI), a program of regular F.VIII therapy, is used to eradicate the inhibitor. A landmark study demonstrated ITI was equally effective with high-dose (HD) (200 IU/kg/day) or low-dose (LD) (50 IU/kg 3x/week) F.VIII, but found a bleeding rate 2-fold greater in the LD arm. As the inhibitor neutralizes F.VIII, no difference in bleeding was expected. We hypothesized that thrombin generation assay (TGA), a global measure of tissue factor-induced thrombin generation that predicts bleeding in hemophilia better than F.VIII, might clarify this question.
Methods: Following IRB approval, TGA was performed on thawed, re-calcified CTI /citrate plasma samples from the ITI repository, using re-lipidated tissue factor and a BioTek Synergy 4 fluorescence plate reader. TGA parameters, i.e. peak thrombin (peak T), estimated thrombin potential (ETP), maximum rate (MaxR), lag time (LAG), were compared by dose group, and repeated after F.VIII spike to neutralize the inhibitor and enhance TGA sensitivity. Significance was calculated by fitting generalized estimating equations linear models to account for serial measurements, with log-transformed TGA as outcome and dose group as predictor. Wilcoxon rank sum test was used to compare bleeds by dose group. Spearman correlation was used to compare TGA and bleeds, and TGA and inhibitor titer. The first TGA sample per subject was used in the analysis; and, separately, all TGA samples per subject. Sensitivity analysis was performed by 95% bootstrap confidence intervals to account for multiple measurements per subject. Total F.VIII antibody (NNA+NA) was analyzed by fluorescent immunoassay (FLI) and compared with anti-VIII (BU/ml) by Pearson correlation.
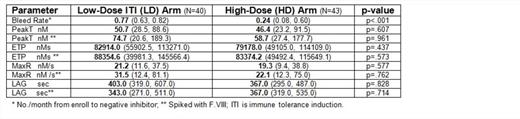
Results: Samples (n=83) from 31 subjects, 14 LD and 17 HD, were available. There was no difference in peak T, ETP, MaxR, or LAG between LD and HD; after VIII spike (Table); nor between non-tolerized (n=56) and tolerized (n=27) subjects, all p>.05. There was no correlation between TGA and bleeding, nor between TGA and anti-VIII titer, all p>.05. In 19 subjects in whom anti-VIII fell to <1.0 BU, including 12 (70.6%) HD and 7 (50.0%) LD, the NNA+NA prevalence was 17/19 (89.5%). Anti-VIII titer and total F.VIII antibody (NNA+NA) were highly correlated, r=.807, p<.001.
Discussion: Despite ITI, the majority of inhibitor patients have detectable non-neutralizing inhibitors. Further, TGA is detectable despite the presence of inhibitors, but does not predict bleeding by ITI dose arm, suggesting thrombin generated after F.VIII dosing may be too low or too short-lived to detect. Limitations include the small sample size and random post-F.VIII sampling. Future studies should evaluate TGA prospectively, early after dosing to determine if thrombin generation predicts bleeding in inhibitor patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal