Abstract
Background: Mantle cell lymphoma (MCL) is incurable with chemo-immunotherapy. Epigenetic modifications including altered DNA methylation and histone acetylation previously identified in MCL has provided the rationale for using the combination of a hypomethylating agent and a histone deactylase inhibitor. We aimed to study a combination of agents with differing mechanisms of action including vorinostat (SAHA), cladribine, and rituximab (SCR) in lymphoid malignancies.
Methods: In this single arm, open label, investigator initiated Phase 1-2 study performed at two centers, we enrolled 65 patients with non-Hodgkin’s lymphomas (NHL) including diffuse large B-cell lymphoma, chronic lymphocytic leukemia (CLL), indolent NHL, and mantle cell lymphoma (MCL). All patients were treated with cladribine 5 mg/m2 IV (days 1-5), rituximab 375 mg/m2 IV (weekly x 4 for cycle 1 and 1x/month) and vorinostat orally once daily (days 1-14) every 28 days for up to 6 cycles. All responding patients were eligible for Rituximab maintenance every 2-3 months as part of standard of care treatment per investigator discretion. The phase 1 portion of the study enrolled patients with relapsed disease in a standard 3+3 dose escalation design using 3 dose levels of vorinostat 200 mg, 300 mg, 400 mg). The primary endpoint of the phase 1 study was dose limiting toxicity (DLT). Phase 2 (n = 55) included relapsed patients (n= 17) with MCL, CLL, FL, and previously untreated MCL (n=38). Primary objectives for phase 2 were objective response rate (ORR), tolerability, and toxicity. Secondary endpoints included progression-free survival (PFS) and overall survival (OS). The analysis was done by intent to treat. Scientific correlative studies included analysis of CD20 expression, histone acetylation, gene microarray, qRT-PCR and HELP methylation analysis. This study is registered with clinicaltrials.gov (NCT00764517).
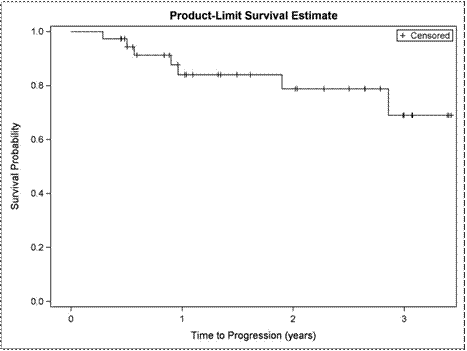
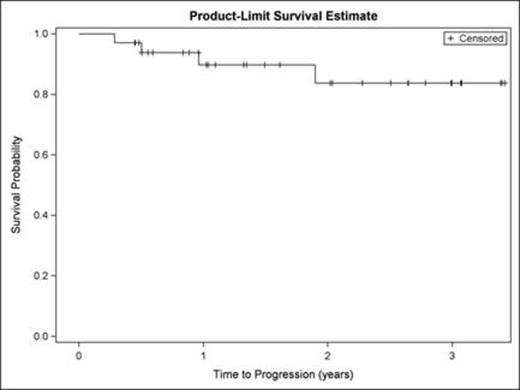
Findings:The median age for phase 1 and 2 was 64 (range 45-84). No DLTs were seen in the Phase 1 and the achieved phase 2 starting dose was vorinostat 400 mg po (days 1-14) combined with cladribine 5mg/m2 IV (days 1-5), and rituximab 375 mg/m2 (weekly x 4 for cycle 1 and 1x/month for cycles 2-6). For Phase 2, the ORR (CR + PR) for relapsed patients was 47% (8/17). For the previously untreated MCL cohort (n=38), 33 (87%) of subjects were male, 36 (95%) had stage 4 disease, and 7 (19%) had blastoid histology. 11 (29%) had a high MIPI. ITT ORR in this cohort was 97% (37/38) with 31/37 (84%) attaining a CR-one patient was not evaluable due to an infusion reaction necessitating removal from the study during cycle 1. CR was achieved in 31/34 (91%) of patients without blastic MCL.The median PFS and OS for the relapsed cohort were 25 months and 25.7 months respectively. For the previously untreated cohort, at a median follow up of 18.7 months (.36-41 months), 7 patients had relapsed and 4 had died. 3 of the 7 relapses occurred in patients with blastoid MCL. Of the 4 deaths, 1 patient died due to a cardiac event while in CR. The median PFS (Figure 1A) and OS (Figure 1B) for this previously untreated cohort could not be estimated because the curves did not reach the .5 level. PFS and OS will be updated at the meeting.
Primary ≥ 3 toxicities (%) for phase 1 and 2, irrespective of drug attribution/causality, were hematologic. Despite the high frequency of neutropenia, ≥ grade 3 neutropenic fever or infections were relatively low (<10%). Fatigue was the most frequent ≥ 3 non-hematologic toxicity. There was one death due to pulmonary hemorrhage in a patient with relapsed MCL with significant pulmonary involvement.
Correlative studies showed that treatment with this regimen resulted in dramatic changes in mRNA expression of multiple tumor suppressor genes including DUSP1, DUSP 2, and p53, as well as changes in CD20 mRNA levels, but increases were not uniform and did not correlate with response or outcome. Conversely, differences in cyclin D1 genotype known to encode a splice variant that is associated with increased risk of multiple cancers as well as nuclear localization of the protein, was associated with blastic histology and found to be inversely related to response.
Conclusions: The SCR regimen is an active and well tolerated regimen, especially in patients with previously untreated MCL. Similar to other current MCL induction regimens, patients with MCL blastoid variant appear to have inferior outcomes.
Spurgeon:Merck Pharmaceuticals: Research Funding. Off Label Use: vorinostat off label for the treatment of CLL and NHL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal