Abstract
Background: PV is a myeloproliferative neoplasm characterized by increased red cell mass and elevated hematocrit. Phlebotomy represents the initial treatment option to lower hematocrit with the goal of reducing the risk of thrombosis. However, many patients require cytoreductive therapy, HU being the most commonly used treatment. Based on European LeukemiaNet (ELN) guidelines, response to PV therapies includes clinicohematologic response (CHR) which is based on several laboratory parameters (hematocrit values, platelet count, white blood cell count) as well as disease-related symptoms. The aim of this study was to investigate the treatment patterns, outcomes, and unmet medical needs among patients with PV treated with HU in a real-world setting.
Methods: A retrospective chart review of PV patients was conducted in the United States between April-July 2014. Oncologists and hematologists abstracted data from patient charts into an online survey. Physicians were eligible to participate if they spent ≥50% of their time on direct patient care and had ≥5 PV patients under their care in the past 12 months with at least 25% of whom had prior (if not current) HU experience. Initial individual qualitative interviews with a subset of eligible physicians (N=19) were conducted to inform the design of the survey instrument. A pilot test survey with 28 physicians meeting eligibility was conducted to demonstrate feasibility and included in the final analyses. Inclusion criteria for patient charts were: age≥18 years, alive at time of chart abstraction or deceased within the past 6 months, diagnosed with PV 3-15 years ago, received HU therapy for ≥2 months within the last 5 years, had medical record data 12 months pre- and post-HU initiation, and were not part of a PV-related clinical trial. Treatment history, lab values, disease symptoms, and healthcare resource utilization data were collected and reported descriptively.
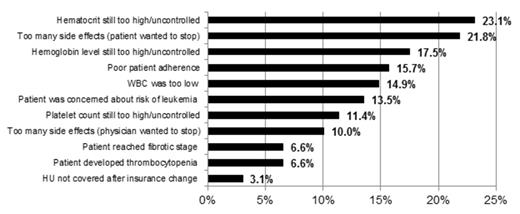
Results: A total 329 physicians participated (Hem Oncs=78.1%, Med Oncs=15.5%, and hematologists=6.4%) and provided information on 1309 PV patients. Almost two-thirds (62.3%) of patients were male, mean age was 62.5 years (SD=12.2), and mean time since diagnosis was 5.2 years (SD=2.8). Among those currently on HU therapy (n=1,080; 82.5%), mean duration of therapy was 47.0 months (SD=30.8) and mean daily dose was 984 mg (SD=674 mg). A total of 229 (17.5%) of patients had discontinued HU therapy. Prior to discontinuing HU treatment, mean therapy duration was 23.2 months (SD=24.5), and mean daily dose immediately prior to discontinuing was 991 mg (SD=689 mg); 27.3% (n=183) of patients had a dose adjustment in the 3 months prior to discontinuing (range: 1-8 adjustments). The most common reasons for HU discontinuation were elevated hematocrit (23.1%) and the presence of drug-related side effects (21.8%) (Figure 1 ). Among those currently on HU, a significant proportion had elevated blood counts above ELN response thresholds: 34.4% had hematocrit level ≥45%, 59.4% had platelet levels >400x109/L, and 58.2% had WBC >10x109/L. Two-thirds (66.3%) of patients had at least one elevated value, 40.3% had at least two elevated values, and 19.8% had all three elevated. The most commonly observed PV-related signs and symptoms were fatigue (62.2%) and splenomegaly (57.3%). Furthermore, among patients currently on HU therapy, almost half (46.2%) experienced new PV-related symptoms in the past 12 months, the most common of which fatigue (22.7%) and splenomegaly (19.5%) in the past 12 months.
Conclusions: In our study, a significant number of patients with PV discontinued HU therapy due to a lack of effectiveness or tolerability. Of those still on HU therapy, the majority did not achieve ELN response criteria for CHR. Furthermore, nearly half of the patients experienced new PV-related symptoms fatigue and splenomegaly despite HU treatment. Consistent with other reports, these study findings demonstrate that despite HU treatment, many patients continue to have uncontrolled PV. These data further support the significant unmet medical need in PV, including the need for more effective treatment options.
Reasons for discontinuation among those who discontinued HU (N=229).
Parasuraman:Incyte Corporation: Employment, Equity Ownership. Off Label Use: Ruxolitinib is a JAK1/JAK2 inhibitor approved for the treatment of patients with intermediate or high-risk myelofibrosis, including primary myelofibrosis, post polycythemia vera myelofibrosis, and post-essential thrombocythemia myelofibrosis. DiBonaventura:Incyte Corporation: Research Funding. Reith:Incyte Corporation: Employment, Equity Ownership. Concialdi:Incyte Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal