Abstract
Mutations affecting the spliceosomal protein U2AF1 are among the most common mutations observed in patients with MDS and related disorders. However, it is unclear how these mutations affect the normal RNA splicing process, and how the resulting changes in splicing contribute to myeloid dysplasia. Here, we combined the strengths of data from primary AML patient samples with the controlled context of isogenic cell lines. We generated K562 erythroleukemic cell lines stably expressing each of the four common U2AF1 mutations (S34F, S34Y, Q157P, and Q157R). We compared expression of each of these mutant alleles with knock down of endogenous U2AF1 to compare the relative consequences of U2AF1 mutations versus loss of function.
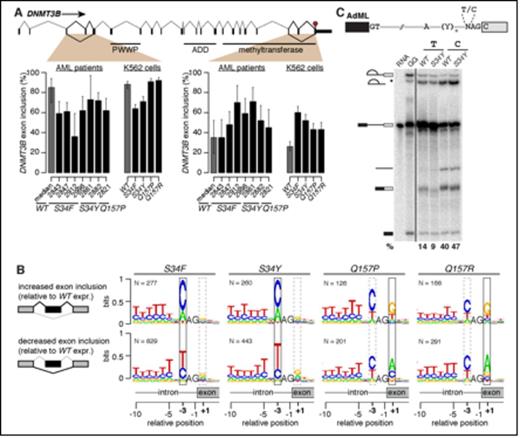
We first sought to identify changes in splicing driven by U2AF1 mutations that contribute to myeloid dysplasia. We compared the splicing of ~125,000 annotated alternative splicing events and ~160,000 constitutively spliced junctions between AML samples with or without mutations (TCGA cohort), as well as our isogenic K562 cell lines stably expressing either mutant (S34F, S34Y, Q157P, and Q157R) or wild-type (WT) alleles of U2AF1. Unsupervised cluster analysis revealed that S34F/Y versus Q157P/R samples clustered together in both the AML data and our cell lines, suggesting that U2AF1 mutations affecting different residues of the protein have different molecular consequences. Intersecting the AML and K562 data, we identified >300 splicing events that were consistently differentially spliced in association with S34 mutations, and a similar number for Q157 mutations. Many of these splicing events affected biological pathways that have been implicated in myeloid malignancies, including DNA methylation (DNMT3B), X chromosome inactivation (H2AFY), the DNA damage response (ATR, FANCA), and apoptosis (CASP8). For example, two exons of DNMT3B are differentially spliced in both AML samples and our K562 cells (Figure A), including an exon lying within the methyltransferase domain.
We next identified mechanistic changes in the splicing process caused by U2AF1 mutations. U2AF1 binds the intron-exon boundary by sequence-specifically recognizing the AG dinucleotide and flanking sequence positions that define the 3' splice site. Comparing AML samples and K562 cells with and without U2AF1 mutations, we found that S34 and Q157 mutations give rise to specific and distinct alterations in 3' splice site preference. S34 mutations alter the consensus nucleotide immediately before the AG dinucleotide, while Q157 mutations alter the consensus nucleotide immediately after the AG (Figure B). We observed highly similar allele-specific alterations in 3' splice site preference in every AML patient with a U2AF1 mutation, as well as all K562 cell lines expressing a U2AF1 mutant allele. In contrast, knock down of endogenous U2AF1 caused no alterations in the consensus sequence at those positions, indicating that U2AF1 mutations do not cause loss of function at the level of RNA splicing.
To confirm that the nucleotides immediately before and after the AG determine whether a splice site responds to U2AF1 mutations, we created minigenes of cassette exons within the ATR and EPB49 genes. We found that response to U2AF1 S34 and Q157 mutations requires the endogenous nucleotides immediately before and after the AG, as predicted by our genomics analysis, and that mutating these positions abolishes response to U2AF1 mutations. Finally, we recapitulated the RNA splicing process in vitro using nuclear extract from blood cells expressing either wild-type or mutant U2AF1 to show that identical changes in splice site preference occur in a controlled in vitro context (Figure C).
Together, our data show that U2AF1 mutations cause allele-specific alterations in normal 3' splice site recognition in patients, in isogenic cell lines, and in vitro. These alterations in splice site preference give rise to mis-splicing that affects many genes previously implicated in myeloid malignancies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal