Abstract
CLL is a highly heterogeneous disorder with some patients developing progressive disease shortly after diagnosis while other patients have stable disease for decades. In addition to established clinical prognosticators, recent genomic studies have identified that intra-tumoral genetic heterogeneity (the presence of multiple subclones within an individual patient), is also correlated with adverse outcome. However, few studies have performed longitudinal sequencing of uniform cohorts of CLL patients to delineate the contribution of subclonal events to CLL progression. Here we performed extensive DNA and RNA-sequencing of 208 CLL samples from 69 CLL patients over time (average of 3.2 samples/patient), all of whom initially presented with untreated CLL without indication for therapy. The goals of this effort were to identify genomic alterations (1) predictive of disease progression at time of initial presentation and (2) enriched in serial samples with disease progression.
Of the 69 patients, 49 patients developed progressive disease requiring initiation of therapy ("progressors") while 20 did not progress over a similar period of observation ("non-progressors"). We sequenced the entire coding region of 405 genes known to be mutated in cancer as well as the RNA of 265 genes involved in fusions. Adaptor ligated sequencing libraries were captured by solution hybridization and sequenced with Illumina HiSeq in a CLIA-certified laboratory. Median exon coverage for DNA sequencing was 466X (range 315-714X) and >20,000,000 unique pairs for RNA sequencing. The average period between initial and serial sample was 589 days; the median clinical follow-up following last sample was 1,045 days. All clinical variables including IGHV mutational status, cytogenetics/FISH were known for each patient and progressor and non-progressor samples had identical frequencies of IGHV unmutated patients.
Analysis of the overall cohort across all samples identified mutations in 78 genes with the 10 most mutated being NOTCH1 (30.4% of patients), KRAS (24.6%), SF3B1 (20.3%), TP53 (18.8%), ATM (15.9%), FBXW7 (10.1%), BCOR (8.7%), XPO1 (7.2%), MLL2 (7.2%), BRAF (5.8%), and AXIN1 (5.8%). Paired bone marrow (BM) and peripheral blood (PB) CLL cells were sequenced in 15 patients. Interestingly, the constellation of genomic alterations differed between BM and PB in one-third of cases, and the variant allele frequency (VAF) of mutations common to both compartments differed by >10% in 60% of patients.
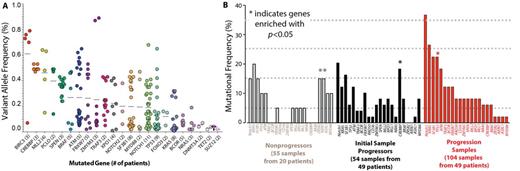
Frequent mutations were identified in genes not previously described to be mutated at a rate >5% in CLL. For example, mutations activating MAP kinase signaling (KRAS, BRAF, NRAS, and MAP2K1) were identified in 24% of the cohort and largely occurred in a mutually exclusive manner. The majority of MAP kinase pathway mutations were subclonal with a median VAF of 5% (range 1-47%) (Figure A), possibly explaining why these mutations have not been appreciated in prior studies at lower sequencing depth. Additional recurrent mutations not previously described as recurrent in CLL but identified here, included loss-of-function mutations in AXIN1, a negative regulator of WNT/b catenin signaling, and SPEN, a transcriptional regulator that opposes Notch signaling.
Comparison of mutational frequencies in non-progressors (55 samples from 20 patients), initial samples in progressors (54 samples from 49 patients), and progression samples (104 samples from 49 patients) revealed clear differences in the somatic mutations present at each state (Figure B). Mutations in TP53 and CREBBP were significantly enriched in progressors while mutations in AXIN1 and ZRSR2 were enriched in non-progressors.
We next attempted to identify changes in clonal architecture with progression. Using a comprehensive sequencing approach in a CLIA-certified CAP-accredited laboratory, we were able to identify mutations occurring with disease progression in 24/49 patients who developed disease progression, some of which were potentially clinically actionable. In contrast, only 2 of 31 non-progressor patients developed new genomic alterations in serial samples (p<0.0001).
The high-depth comprehensive combined DNA and RNA sequencing approach utilized here in an initially untreated CLL cohort with serial samples delineated genomic predictors associated with disease progression and underscore the utility of this strategy to detect clonal evolution in CLL.
Nahas:Foundation Medicine Inc.: Employment. He:Foundation Medicine: Employment. Donahue:Foundation Medicine: Employment. Otto:Foundation Medicine, Inc.: Employment. Dogan:Foundation Medicine, Inc: Consultancy. Lipson:Foundation Medicine, Inc.: Employment. van den Brink:Foundation Medicine, Inc: Consultancy. Miller:Foundation Medicine, Inc: Employment. Abdel-Wahab:Foundation Medicine Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal